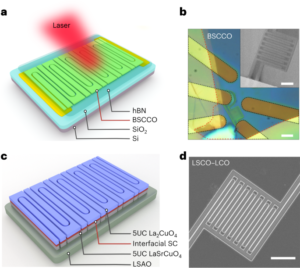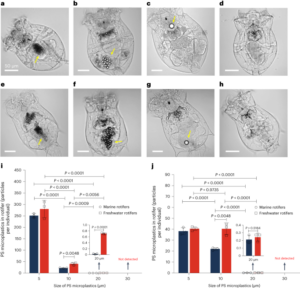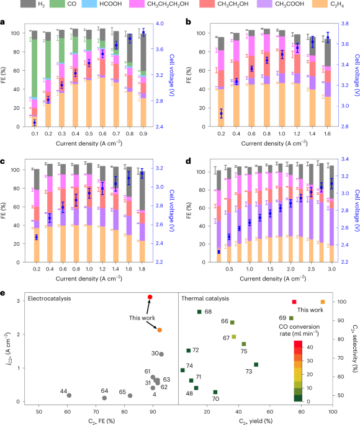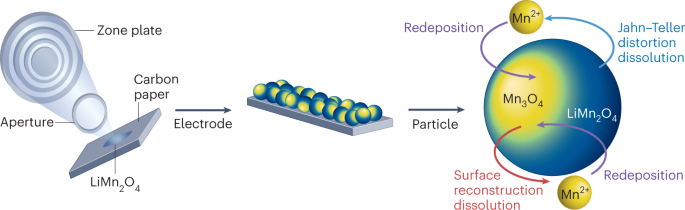
Among Li-ion battery components, the cathode materials contribute substantially to the cost due to a large fraction of high-value metals such as cobalt (Co) and nickel (Ni). Due to economic considerations, these metals have been substituted by the highly abundant manganese (Mn). Unfortunately, this element substitution sacrifices the cycle life of the cathode, as it intensifies side reactions such as transition metal (TM) dissolution. While various research has established the correlation between Mn dissolution and the loss of electrochemical performance, a clear description of the mechanism of Mn dissolution is yet to emerge. Now, writing in Nature Nanotechnology, Zhang et al. have employed X-ray fluorescence microscopy (XFM) to visualize and quantify the particle-/electrode-level in situ Mn dissolution/redeposition (D/R) dynamics upon cycling and elucidate the corresponding mechanism1.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Minting the Future w Adryenn Ashley. Access Here.
- Buy and Sell Shares in PRE-IPO Companies with PREIPO®. Access Here.
- Source: https://www.nature.com/articles/s41565-023-01396-1
- :has
- :is
- 1
- 2023
- a
- abundant
- AL
- Anchor
- and
- AS
- battery
- been
- between
- Bottom
- by
- clear
- click
- CO
- components
- considerations
- contribute
- Correlation
- Corresponding
- Cost
- cycle
- description
- due
- dynamics
- E&T
- Economic
- element
- emerge
- employed
- established
- Ether (ETH)
- fraction
- getting
- Have
- highly
- HTTPS
- in
- Intensifies
- IT
- large
- Life
- LINK
- loss
- materials
- mechanism
- metal
- Metals
- Microscopy
- nanotechnology
- Nature
- Nickel
- now
- of
- performance
- plato
- Plato Data Intelligence
- PlatoData
- reactions
- research
- side
- substantially
- such
- The
- These
- this
- TM
- to
- transition
- unfortunately
- upon
- various
- while
- writing
- x-ray
- yet
- zephyrnet