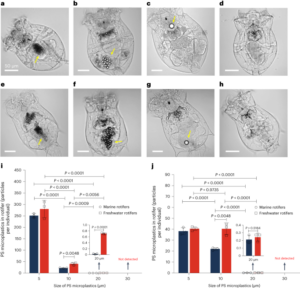
Graphene, transition-metal dichalcogenides, MXenes and the other members of the flatland family are becoming a rich playground for chemists, enlarging the range of applications these nanomaterials can be used for.
The exfoliation of graphite into 2D sheets, 20 years ago, was a momentous point in the history of nanotechnology. The experiments by Geim and Novoselov on graphene (recognized in 2010 with the physics Nobel Prize) were the first ones that tap into the new and rich vein of 2D materials, a vein that keeps on giving today more than ever before. Initially a playground for physicists, chemists are nowadays contributing with their knowledge of synthetic methodologies, functionalization protocols, purification procedures, and spectroscopic characterization tools. Chemistry of 2D materials has become a lively research topic and the subject of several international conferences.
Credit: Science History Images / Alamy Stock Photo
After graphene, the family of 2D materials grew to include systems with different and complementary properties: electrical insulators, like hexagonal BN; or semiconductors, like black phosphorus or molybdenum disulfide. More exotic electronic, optical and thermal properties could recently be achieved when stacking 2D material in van der Waals structures of different stacking orders and angles.
Chemists joined with the successful preparation of 2D polymers. These are very pleasing nanomaterials owing to their atomically controlled structure from the bottom-up, but for the non-polymer chemist, these structures have remained largely inaccessible.
The first chemists to adopt 2D materials started using graphene and graphene oxide to protect unstable interfaces in energy devices. In batteries for example, the solid–electrolyte interface between the anode and the electrolyte can now be engineered to control the flow of lithium ions without the two phases reacting, which would cause performance degradation. In photovoltaics, and especially in perovskite solar cells, performances directly depend by the stability of the perovskite material and how the interfaces with the neighbouring layers let holes and electrons through. The most coveted solution is to find a material that can isolate the functional layer, but not add weight or resistance to the flow of charged species. 2D materials have therefore been an obvious choice for these communities.
In heterogeneous catalysis, 2D materials are an attractive system for the simple reason that catalysis occurs on surfaces and all atoms in 2D materials are in principle part of the surface. There are no bulk atoms.
Another active area is using 2D materials for membranes to purify gases or liquids. Of particular note is the effort on water purification and desalination, where the specificity of the interaction with certain compounds (for instance, per- and polyfluoroalkyl substances, or PFAS) and ions can result in selective adsorption and removal. Some 2D materials-enriched polymeric fibres and foams have reached the pilot plants stage for purifying waste waters.
There are probably two main thrusts where chemists can make a substantial contribution in the field of 2D materials: synthesis and functionalization.
The bottleneck to commercialization remains the difficulty of scaling up the production of well-characterized, functionalized 2D materials, without losing their lab-scale properties. Ideally, synthetic methodologies should be solution-based, methodologies that chemists are certainly conversant with. The chemical approach should be able to achieve large-scale control over thickness, size, crystal phase and all the relevant parameters for the application at hand. 2D materials-based inks for printing devices onto flexible substrates also represent a cost-effective approach for large-scale production.
Regarding functionalization, graphene oxide and reduced graphene oxide are relatively easy to work with, due to the presence of reactive oxygenated species, such as epoxides and carboxylic groups. Other materials are more particular: if there are no defects, they may require a pre-treatment before they can be grafted; some, intrinsically behave like Lewis acids or Lewis bases, owing to their dangling orbitals. Successful functionalization of 2D materials has already been demonstrated to produce chemo- and bio-sensors, in which the interaction with targeted analyte modifies the electronic or optical properties of the 2D material, which can then be detected. 2D materials-based biosensors can be extremely flexible and adhere well to the skin, for example for non-invasive and continuous monitoring of biomarkers in sweat.
Be it for energy, water or health, one can nowadays draw a continuum between fundamental 2D materials research and devices for applications that span beyond what initially was a physicists’ playground. Such is the pulling force of nanoscience and technology: a convergence of expertise aiming to tackle complex and pressing societal problems from various disciplinary angles in a concerted, interdisciplinary effort.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- EVM Finance. Unified Interface for Decentralized Finance. Access Here.
- Quantum Media Group. IR/PR Amplified. Access Here.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.nature.com/articles/s41565-023-01433-z
- :has
- :is
- :not
- :where
- $UP
- 20
- 20 years
- 2D
- 2D materials
- 300
- 32
- 39
- 40
- a
- Able
- Achieve
- achieved
- active
- add
- adhere
- adopt
- ago
- Aiming
- All
- already
- also
- an
- and
- Application
- applications
- approach
- ARE
- AREA
- AS
- At
- attractive
- batteries
- BE
- become
- becoming
- been
- before
- between
- Beyond
- biomarkers
- Black
- but
- by
- CAN
- Cause
- Cells
- certain
- certainly
- charged
- chemical
- chemistry
- Chemists
- choice
- commercialization
- Communities
- complementary
- complex
- concerted
- conferences
- continuous
- Continuum
- contributing
- contribution
- control
- controlled
- Convergence
- cost-effective
- could
- coveted
- credit
- Crystal
- demonstrated
- detected
- Devices
- different
- Difficulty
- directly
- disciplinary
- draw
- due
- easy
- effort
- electrolyte
- Electronic
- electrons
- energy
- especially
- EVER
- example
- Exotic
- experiments
- expertise
- extremely
- family
- field
- Find
- First
- flexible
- flow
- For
- Force
- from
- functional
- fundamental
- Giving
- Graphene
- Group’s
- hand
- Have
- Health
- history
- Holes
- How
- HTTPS
- if
- images
- in
- inaccessible
- include
- initially
- instance
- interaction
- Interface
- interfaces
- International
- into
- intrinsically
- IT
- joined
- knowledge
- large-scale
- largely
- layer
- layers
- Lewis
- like
- lithium
- losing
- Main
- make
- material
- materials
- Matter
- May..
- Members
- methodologies
- monitoring
- more
- most
- Nanomaterials
- nanotechnology
- Nature
- New
- no
- nobel prize
- now
- obvious
- of
- on
- ONE
- ones
- or
- orders
- Other
- over
- parameters
- part
- particular
- performance
- performances
- phase
- Physics
- pilot
- plants
- plato
- Plato Data Intelligence
- PlatoData
- Point
- Polymers
- preparation
- presence
- pressing
- principle
- printing
- prize
- probably
- problems
- procedures
- produce
- Production
- properties
- protect
- protocols
- pulling
- range
- reached
- reason
- recently
- recognized
- Reduced
- relatively
- relevant
- remained
- remains
- removal
- represent
- require
- research
- Resistance
- result
- Rich
- scaling
- Science
- selective
- Semiconductors
- several
- should
- Simple
- Size
- Skin
- societal
- solar
- Solar cells
- solution
- some
- span
- specificity
- Stability
- stacking
- Stage
- started
- stock
- structure
- subject
- substantial
- successful
- such
- Surface
- SWEAT
- synthetic
- system
- Systems
- tackle
- Tap
- targeted
- Technology
- than
- that
- The
- their
- then
- There.
- therefore
- thermal
- These
- they
- Through
- to
- today
- tools
- topic
- two
- used
- using
- various
- very
- was
- Waste
- Water
- Waters
- weight
- WELL
- were
- What
- when
- which
- with
- without
- Work
- would
- years
- zephyrnet