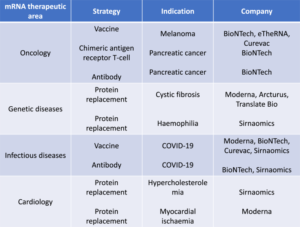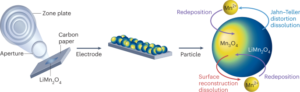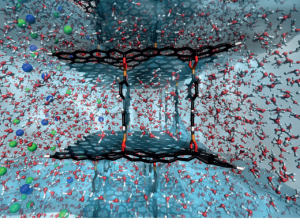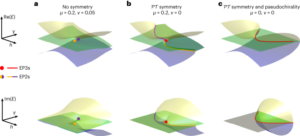
Frank, J. A., Antonini, M.-J. & Anikeeva, P. Next-generation interfaces for studying neural function. Nat. Biotechnol. 37, 1013–1023 (2019).
Machado, T. A., Kauvar, I. V. & Deisseroth, K. Multiregion neuronal activity: the forest and the trees. Nat. Rev. Neurosci. 23, 683–704 (2022).
Logothetis, N. K. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012).
Gradinaru, V. et al. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Fernández-Ruiz, A. et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 372, eabf3119 (2021).
Bi, X.-a et al. A novel CERNNE approach for predicting Parkinson’s disease-associated genes and brain regions based on multimodal imaging genetics data. Med. Image Anal. 67, 101830 (2021).
Zhang, D. et al. Multimodal classification of Alzheimer’s disease and mild cognitive impairment. Neuroimage 55, 856–867 (2011).
Chiarelli, A. M. et al. Deep learning for hybrid EEG-fNIRS brain–computer interface: application to motor imagery classification. J. Neural Eng. 15, 036028 (2018).
Halme, H.-L. & Parkkonen, L. Across-subject offline decoding of motor imagery from MEG and EEG. Sci. Rep. 8, 10087 (2018).
Liu, X. et al. Decoding of cortex-wide brain activity from local recordings of neural potentials. J. Neural Eng. 18, 066009 (2021).
Siegle, J. H. et al. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 592, 86–92 (2021).
Kuzum, D. et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 5, 5259 (2014).
Park, D.-W. et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5, 5258 (2014).
Thunemann, M. et al. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat. Commun. 9, 2035 (2018).
Driscoll, N. et al. Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale. Commun. Biol. 4, 136 (2021).
Park, D.-W. et al. Electrical neural stimulation and simultaneous in vivo monitoring with transparent graphene electrode arrays implanted in GCaMP6f mice. ACS Nano 12, 148–157 (2018).
Ledochowitsch, P. et al. A transparent μECoG array for simultaneous recording and optogenetic stimulation. In Proc. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2937–2940 (IEEE, 2011).
Kwon, K. Y. et al. Opto-μECoG array: a hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics. IEEE Trans. Biomed. Circuits Syst. 7, 593–600 (2013).
Kunori, N. & Takashima, I. A transparent epidural electrode array for use in conjunction with optical imaging. J. Neurosci. Methods 251, 130–137 (2015).
Ledochowitsch, P. et al. Strategies for optical control and simultaneous electrical readout of extended cortical circuits. J. Neurosci. Methods 256, 220–231 (2015).
Zhang, J. et al. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett. 18, 2903–2911 (2018).
Chen, Z. et al. Flexible and transparent metal nanowire microelectrode arrays and interconnects for electrophysiology, optogenetics, and optical mapping. Adv. Mater. Technol. 6, 2100225 (2021).
Neto, J. P. et al. Transparent and flexible electrocorticography electrode arrays based on silver nanowire networks for neural recordings. ACS Appl. Nano Mater. 4, 5737–5747 (2021).
Araki, T. et al. Long‐term implantable, flexible, and transparent neural interface based on Ag/Au core–shell nanowires. Adv. Healthc. Mater. 8, 1900130 (2019).
Seo, K. J. et al. Transparent electrophysiology microelectrodes and interconnects from metal nanomesh. ACS Nano 11, 4365–4372 (2017).
Seo, J. W. et al. Artifact‐free 2D mapping of neural activity in vivo through transparent gold nanonetwork array. Adv. Funct. Mater. 30, 2000896 (2020).
Obaid, S. N. et al. Multifunctional flexible biointerfaces for simultaneous colocalized optophysiology and electrophysiology. Adv. Funct. Mater. 30, 1910027 (2020).
Qiang, Y. et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 4, eaat0626 (2018).
Cho, Y. U. et al. Ultra‐low cost, facile fabrication of transparent neural electrode array for electrocorticography with photoelectric artifact‐free optogenetics. Adv. Funct. Mater. 32, 2105568 (2022).
Yang, W. et al. A fully transparent, flexible PEDOT:PSS–ITO–Ag–ITO based microelectrode array for ECoG recording. Lab Chip 21, 1096–1108 (2021).
Kshirsagar, P. et al. Transparent graphene/PEDOT:PSS microelectrodes for electro‐ and optophysiology. Adv. Mater. Technol. 4, 1800318 (2019).
Viswam, V. et al. Optimal electrode size for multi-scale extracellular-potential recording from neuronal assemblies. Front. Neurosci. 13, 385 (2019).
Rogers, N. et al. Correlation structure in micro-ECoG recordings is described by spatially coherent components. PLoS Comput. Biol. 15, e1006769 (2019).
Harris, K. D. et al. Improving data quality in neuronal population recordings. Nat. Neurosci. 19, 1165–1174 (2016).
Akinwande, D. et al. A review on mechanics and mechanical properties of 2D materials—graphene and beyond. Extrem. Mech. Lett. 13, 42–77 (2017).
Kireev, D. et al. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 17, 864–870 (2022).
Sahni, D. et al. Biocompatibility of pristine graphene for neuronal interface. J. Neurosurg. Pediatr. 11, 575–583 (2013).
Liu, X. et al. E-cannula reveals anatomical diversity in sharp-wave ripples as a driver for the recruitment of distinct hippocampal assemblies. Cell Rep. 41, 111453 (2022).
Ding, D. et al. Evaluation of durability of transparent graphene electrodes fabricated on different flexible substrates for chronic in vivo experiments. IEEE Trans. Biomed. Eng. 67, 3203–3210 (2020).
Wilson, M. N. et al. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat. Commun. 13, 7945 (2022).
Bonaccini Calia, A. et al. Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 17, 301–309 (2022).
Masvidal-Codina, E. et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 18, 280–288 (2019).
Lu, Y. et al. Ultralow impedance graphene microelectrodes with high optical transparency for simultaneous deep two‐photon imaging in transgenic mice. Adv. Funct. Mater. 28, 1800002 (2018).
Xia, J. et al. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 4, 505–509 (2009).
Liu, X. et al. Multimodal neural recordings with Neuro-FITM uncover diverse patterns of cortical–hippocampal interactions. Nat. Neurosci. 24, 886–896 (2021).
Łęski, S. et al. Frequency dependence of signal power and spatial reach of the local field potential. PLoS Comput. Biol. 9, e1003137 (2013).
Myers, J. C. et al. The spatial reach of neuronal coherence and spike-field coupling across the human neocortex. J. Neurosci. 42, 6285–6294 (2022).
Liu, X. et al. Decoding ECoG high gamma power from cellular calcium response using transparent graphene microelectrodes. In Proc. 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) 710–713 (IEEE, 2019).
Gallego, J. A. et al. Neural manifolds for the control of movement. Neuron 94, 978–984 (2017).
Elsayed, G. F. et al. Reorganization between preparatory and movement population responses in motor cortex. Nat. Commun. 7, 13239 (2016).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, eaav7893 (2019).
Okun, M. et al. Diverse coupling of neurons to populations in sensory cortex. Nature 521, 511–515 (2015).
Stringer, C. et al. High-dimensional geometry of population responses in visual cortex. Nature 571, 361–365 (2019).
Lin, M. Z. & Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19, 1142–1153 (2016).
Zhang, D. et al. Dealing with the foreign‐body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 31, 2007226 (2021).
Carnicer-Lombarte, A. et al. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front. Bioeng. Biotechnol. 9, 271 (2021).
Salatino, J. W. et al. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Wang, Y. et al. Electrochemical delamination of CVD-grown graphene film: toward the recyclable use of copper catalyst. ACS Nano 5, 9927–9933 (2011).
Brug, G. et al. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 176, 275–295 (1984).
Mayford, M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996).
Wekselblatt, J. B. et al. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Mitani, A. & Komiyama, T. Real-time processing of two-photon calcium imaging data including lateral motion artifact correction. Front. Neuroinform. 12, 98 (2018).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2016).
Yu, B. M. et al. Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. in Advances in Neural Information Processing Systems Vol. 21 (Curran Associates, 2008).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41565-023-01576-z
- :is
- ][p
- 000
- 01
- 07
- 1
- 10
- 11
- 12
- 13
- 14
- 15%
- 16
- 17
- 19
- 1996
- 20
- 2008
- 2011
- 2012
- 2013
- 2014
- 2015
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 2022
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 2D
- 30
- 31
- 32
- 33
- 35%
- 36
- 385
- 39
- 40
- 41
- 43
- 46
- 49
- 50
- 51
- 52
- 53
- 54
- 58
- 60
- 65
- 7
- 75
- 8
- 84
- 9
- 98
- 9th
- a
- across
- activity
- AL
- Alzheimer’s
- analysis
- and
- annual
- Application
- applications
- approach
- Array
- article
- AS
- associates
- At
- b
- based
- behavior
- between
- Beyond
- biology
- Biomaterials
- blood
- Blood Pressure
- body
- Brain
- Brain activity
- by
- Catalyst
- cellular
- classification
- click
- cognitive
- COHERENT
- Communication
- complicated
- components
- Conference
- conjunction
- connection
- constant
- continuous
- control
- Copper
- Correlation
- cortex
- Cost
- data
- data quality
- dealing
- Decoding
- deep
- deep learning
- dependence
- depth
- described
- different
- Disease
- distinct
- diverse
- Diversity
- drive
- driver
- durability
- during
- dynamics
- e
- E&T
- element
- enabled
- encoded
- Engineering
- Ether (ETH)
- evaluation
- experiments
- expression
- extended
- external
- factor
- field
- Film
- flexible
- For
- foreign
- forest
- formation
- Frequency
- from
- fully
- function
- functional
- Genetics
- geometry
- Gold
- Graphene
- hierarchy
- High
- high-resolution
- hippocampal
- http
- HTTPS
- human
- Hybrid
- i
- IEEE
- image
- Imaging
- Impact
- impairment
- improving
- in
- Including
- Indicators
- information
- integrated
- interaction
- interactions
- interconnects
- Interface
- interfaces
- International
- ITS
- large-scale
- learning
- LINK
- local
- Low
- mapping
- materials
- measurement
- mechanical
- mechanics
- Media
- medicine
- Memory
- metal
- mice
- Microscopy
- mild
- monitoring
- motion
- Motor
- mouse
- movement
- nano
- nanotechnology
- Nature
- networks
- Neural
- Neural engineering
- Neural Interface
- neuronal
- Neurons
- New
- next-generation
- Noise
- novel
- of
- offline
- on
- optimal
- patterns
- perception
- periods
- phase
- plato
- Plato Data Intelligence
- PlatoData
- population
- populations
- potential
- potentials
- power
- predicting
- presence
- pressure
- processing
- properties
- quality
- Quantum
- reach
- reaction
- real-time
- recording
- recruitment
- reference
- regions
- regulated
- reorganization
- response
- responses
- reveal
- Reveals
- review
- rhythm
- ripples
- s
- Scholar
- Seizure
- Signal
- Silence
- Silver
- simultaneous
- Size
- Spatial
- standard
- strategies
- structure
- Studying
- Surface
- Survey
- system
- T
- Technology
- The
- Through
- to
- toward
- trans
- Transparency
- transparent
- Trees
- uncover
- use
- using
- via
- visual
- vivo
- W
- with
- X
- zephyrnet