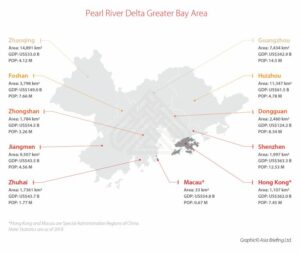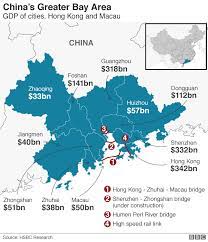
The Greater Bay Area (GBA) – with a total population of approximately 71.2 million people (5% of China’s total population) – includes nine mega cities of Guangdong province: Guangzhou, Shenzhen, Zhuhai, Foshan, Dongguan, Zhongshan, Jiangmen, Huizhou, and Zhaoqing. These nine cities are applicable of urgent use policy for medical devices and drugs starting in May 2021.
While not-yet-approved in the mainland, Hong Kong or Macao approved drugs and medical devices can be utilized in GBA. They can be used only if the significant clinical benefits with urgent needs can be demonstrated. Guangdong provincial NMPA will decide on import approval within 20 working days.
Newly Designated Hospitals
On February 22, 2023, Guangdong Health Commission issued the second batch of applicable institutions to pilot urgent use policy, in which 14 hospitals are included as below; among them, the first five are public tier 3:
- The First Affiliated Hospital of Sun Yat-sen University
- Sun Yat-sen Memorial Hospital, Sun Yat-sen University
- Southern Medical University Nanfang Hospital
- Guangdong Provincial People’s Hospital
- Nansha Hospital, Guangzhou First People’s Hospital
- Guangdong Qifu Hospital
- Guangzhou Ximalin Shunchao Eye Hospital
- Shenzhen Qianhai Shekou Free Trade Zone Hospital
- Shenzhen Hezheng Hospital
- Shenzhen Ximalin Shunchao Eye Hospital
- Zhuhai People’s Hospital (Hengqin Campus)
- Foshan Fosun Zencheng Hospital
- Dongguan Songshan Lake Donghua Hospital
- Dongguan Guangming Eye Hospital
Five hospitals were in the first batch:
- The University of Hong Kong Affiliated Shenzhen Hospital
- Zhongshan Chen Xinghai Hospital
- Guangzhou Modern Hospital
- Guangzhou United Family Hospital
- Zhuhai Simalin Shunchao Eye Hospital
Devices List
The following devices have been designated as urgently needed, in notices published by Guangdong Health Commission:
- bioMerieux: FilmArray Torch (System Base/ Module/Empty Torch Bay Front cover FRU Kit)
- bioMerieux: BioFire BCID2 Panel
- bioMerieux: BioFire Respiratory Panel 2.1(RP2.1)
- bioMerieux: FilmArray Pneumonia Panel Plus (Pneumoplus)
- Medtronic: TYRXTM Absorbable Antibacterial Envelope
- Dutch Ophthalmic Research Center: Posterior segment staining solution
- Dutch Ophthalmic Research Center: Anterior segment staining solution
- Terumo: Thoraflex Hybrid System
- … …
Government Guidance
The Guangdong government released the “Interim Provisions on the Administration of Imported Medicines and Medical Devices in Urgent Clinical Needs in the Guangdong-Hong Kong-Macao Greater Bay Area” on August 27, 2021, finalizing a series of first-of-its-kind measures in China for NMPA unapproved devices.
The provisions include 29 articles, providing clear guidebook for the aspects below:
- Scope of urgent need for medicine and equipment
- Requirements for designated medical institutions
- Review and approval procedure
- Precondition of supplying
- Risk management
- Recall process
If your devices are used in Hong Kong and Macau, and you want to clinically use them in Guangdong, please contact info@ChinaMedDevice.com.
Requirements for Application
The document specifies that the medical devices need to meet the below scenarios to be utilized in Guangdong:
- Urgently needed for clinical use.
- Already be used by public hospitals in Hong Kong and Macau.
- Have significant clinical advantage.
- Have not been approved in Mainland China.
- Have unmet medical needs.
The medical institutes need to meet the requirements as follows:
- They can be sole proprietorship, joint venture, or cooperation.
- Obtained medical institution’s practice license.
- Have robust management system in terms of supply, transportation, storage of medical products.
- Have adverse reaction monitoring agency and emergency plans.
Along with the Interim Provisions are the Submission Materials and Review Guideline. Please email us at info@ChinaMedDevice.com to get an English copy.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://chinameddevice.com/greater-bay-area-hospitals/
- 1
- 2021
- 2023
- a
- Additional
- administration
- ADvantage
- adverse
- Affiliated
- agency
- among
- and
- applicable
- approval
- approved
- approximately
- ARE
- AREA
- articles
- AS
- aspects
- At
- AUGUST
- Bay
- BE
- below
- benefits
- by
- Campus
- CAN
- Center
- chen
- China
- Chinas
- Cities
- clear
- Clinical
- COM
- commission
- contact
- cooperation
- cover
- decide
- demonstrated
- designated
- Devices
- document
- Drugs
- emergency
- English
- eye
- family
- February
- First
- following
- follows
- For
- Free
- front
- GBA
- get
- Government
- greater
- Greater Bay Area
- Guangdong
- Have
- Health
- Hengqin
- Hong
- Hong Kong
- Hospital
- hospitals
- HTTPS
- Hybrid
- import
- in
- include
- included
- includes
- institutions
- Issued
- joint
- joint venture
- jpg
- kit
- Kong
- lake
- License
- Macau
- mainland
- mainland china
- management
- management system
- materials
- measures
- medical
- medical devices
- medicine
- Meet
- Mega
- Memorial
- million
- Modern
- monitoring
- Need
- needed
- needs
- of
- on
- panel
- People
- people’s
- pilot
- plans
- plato
- Plato Data Intelligence
- PlatoData
- please
- plus
- pneumonia
- policy
- population
- practice
- Products
- propelling
- providing
- Provincial
- public
- published
- reaction
- released
- Requirements
- research
- review
- robust
- scenarios
- Second
- segment
- Series
- shenzhen
- significant
- Starting
- storage
- submission
- Sun
- supply
- system
- terms
- that
- The
- Them
- These
- tier
- to
- torch
- Total
- trade
- transportation
- United
- university
- urgent
- us
- use
- utilized
- venture
- which
- will
- with
- within
- working
- Your
- zephyrnet