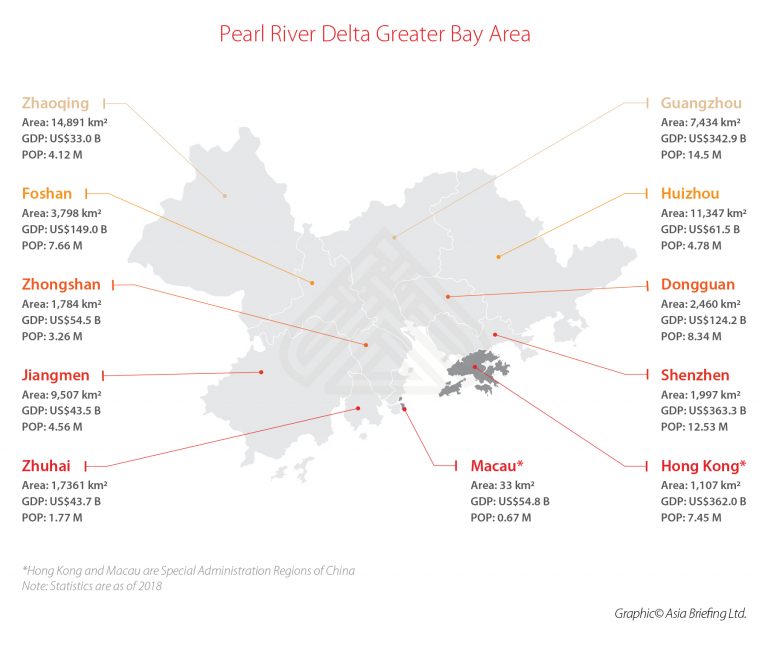
The Greater Bay Area (GBA) – with a total population of approximately 71.2 million people (5% of China’s total population) – includes nine mega cities of Guangdong province: Guangzhou, Shenzhen, Zhuhai, Foshan, Dongguan, Zhongshan, Jiangmen, Huizhou, and Zhaoqing. These nine cities are applicable of urgent use policy for medical devices and drugs starting in May 2021.
While not-yet-approved in the mainland, Hong Kong or Macao approved drugs and medical devices can be utilized in GBA. They can be used only if the significant clinical benefits with urgent needs can be demonstrated. Guangdong provincial NMPA will decide on import approval within 20 working days.
Eight devices as the third batch were enrolled to pioneer the clinical use in GBA on July 7, 2022, adding to the first two batches. For the applicable hospitals to pilot this program, please click HERE
Complete List
Ten devices have been added to the sixth batch of urgently needed devices, according to a notice published by Guangdong Health Commission on January 15, 2024:
- Boston Scientific: FARAWAVE Pulsed Field Ablation Catheter
- Boston Scientific: FARADRIVE Steerable Sheath
- Boston Scientific: FARASTAR Pulsed Field Ablation Generator
- Abbott: AveirTM Leadless Pacemaker
- Abbott: AveirTM Delivery Catheter
- Abbott: AveirTM Introducer
- Abbott: AveirTM Retrieval Catheter
- Medtronic: Heli-FX EndoAnchor System Heli-FX Applier with EndoAnchor Cassette
- Medtronic: Heli-FX Ancillary EndoAnchor Cassette
- Medtronic: Heli-FX EndoAnchor System Heli-FX Guide
Government Guidance
The Guangdong government released the “Interim Provisions on the Administration of Imported Medicines and Medical Devices in Urgent Clinical Needs in the Guangdong-Hong Kong-Macao Greater Bay Area” on August 27, 2021, finalizing a series of first-of-its-kind measures in China for NMPA unapproved devices.
The provisions include 29 articles, providing clear guidebook for the aspects below:
- Scope of urgent need for medicine and equipment
- Requirements for designated medical institutions
- Review and approval procedure
- Precondition of supplying
- Risk management
- Recall process
If your devices are used in Hong Kong and Macau, and you want to clinically use them in Guangdong, please contact info@ChinaMedDevice.com.
Requirements for Application
The document specifies that the medical devices need to meet the below scenarios to be utilized in Guangdong:
- Urgently needed for clinical use.
- Already be used by public hospitals in Hong Kong and Macau.
- Have significant clinical advantage.
- Have not been approved in Mainland China.
- Have unmet medical needs.
The medical institutes need to meet the requirements as follows:
- They can be sole proprietorship, joint venture, or cooperation.
- Obtained medical institution’s practice license.
- Have robust management system in terms of supply, transportation, storage of medical products.
- Have adverse reaction monitoring agency and emergency plans.
For the contract manufacturing policy in GBA, please click HERE
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://chinameddevice.com/urgent-needed-devices-gba/
- :not
- 15%
- 2021
- 2022
- 2024
- 27
- 29
- 7
- a
- According
- added
- adding
- administration
- ADvantage
- adverse
- agency
- and
- applicable
- approval
- approved
- approximately
- ARE
- AREA
- articles
- AS
- aspects
- AUGUST
- Bay
- BE
- been
- below
- benefits
- boston
- by
- CAN
- China
- Chinas
- Cities
- clear
- click
- Clinical
- clinically
- COM
- commission
- contact
- contract
- cooperation
- decide
- delivery
- demonstrated
- designated
- Devices
- document
- Drugs
- emergency
- enrolled
- field
- First
- follows
- For
- GBA
- Government
- greater
- Greater Bay Area
- Guangdong
- Guangzhou
- Have
- Health
- Hong
- Hong Kong
- hospitals
- HTTPS
- if
- import
- in
- include
- includes
- January
- joint
- joint venture
- jpg
- July
- Kong
- License
- Macau
- mainland
- mainland china
- management
- management system
- manufacturing
- May..
- measures
- medical
- medical devices
- medicine
- Meet
- Mega
- million
- monitoring
- Need
- needed
- needs
- nine
- Notice..
- of
- on
- only
- or
- People
- pilot
- pioneer
- plans
- plato
- Plato Data Intelligence
- PlatoData
- please
- please contact
- policy
- population
- practice
- Products
- Program
- providing
- Provincial
- public
- published
- reaction
- released
- Requirements
- retrieval
- robust
- scenarios
- scientific
- Series
- shenzhen
- significant
- sixth
- Starting
- storage
- supply
- system
- terms
- that
- The
- Them
- These
- they
- Third
- this
- to
- Total
- transportation
- two
- unmet
- urgent
- use
- used
- utilized
- venture
- want
- were
- will
- with
- within
- working
- you
- Your
- zephyrnet