Hainan government issued the “Regulations on the Administration of Urgently Needed Imported Drugs and Medical Devices in the Boao Lecheng International Medical Tourism Pilot Zone of Hainan Free Trade Port” on March 28, which will come into force on May 1, 2023.
Urgently Needed Imported Drugs and Medical Devices refer to products that have been approved for marketing overseas, and have not been approved in China, which cannot be replaced by already marketed varieties.
Hainan Boao special zone allows overseas unapproved medical devices, IVDs, drugs, with clinical urgency status, to be used in China. In addition to the overseas medical products being sold and commercialized faster through this special program, overseas manufacturers can apply for RWS to collect RWD as China local clinical evidence to support its national registration approval.
Big names are already on the trial list. It only took four months for Boston Scientific, from submitting the registration application to guaranteeing approval, for its Rezūm water vapor therapy equipment. Allergan, J&J, Edwards Lifesciences, Roche Diagnostics, Advanced Bionics, and Zimmer also used the same pathway to get national approvals.
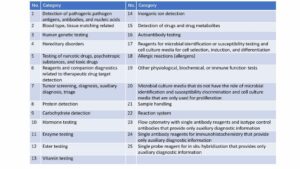
Timeline & Review Focus for Hainan Drugs Medical Devices
Hainan health administration is responsible for assessing the qualifications of medical institutions that apply for import and usage. After accepting the application, the administration shall make a decision of receptance within 10 working days.
After the health administration’s acceptance, it shall issue an assessment opinion within 5 working days.
Hainan NMPA will issue administrative licensing within 7 working days. It will focus on reviewing the overseas listing of the product and adverse events.
Main Responsibility Bearer
Designated medical institutions is responsible for the safety risks of the usage.
The designated institution shall meet four conditions:
- Tier-3 hospital, with departments capable of using the urgent needed drugs and devices.
- Safeguard measures and management systems for circulation, transportation and storage that meet the characteristics and instructions of the drugs and devices.
- Have adverse drug reaction monitoring institution, equipped with full-time personnel and have received professional training.
- Have emergency plans and disposal capabilities for serious adverse reactions/events that may occur in the usage.
The document speculates that the risks of drugs and devices shall be controllable, the source shall be legal, the storage shall be standardized, and the supply pathway shall be clear. It also regulates the circumstances of damage compensation and suspended usage.
View the latest news on Hainan Real World Data program.
View the communication guideline working with regulatory authorities.
View our recorded webinar on Hainan policies.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://chinameddevice.com/hainan-drugs-medical-devices/
- :is
- 1
- 10
- 2023
- 28
- 7
- a
- acceptance
- addition
- administration
- administrative
- advanced
- adverse
- After
- allows
- already
- and
- Application
- Apply
- approval
- approved
- ARE
- AS
- Assessing
- assessment
- BE
- being
- by
- CAN
- cannot
- capabilities
- capable
- characteristics
- China
- Circulation
- circumstances
- clear
- Clinical
- collect
- come
- Compensation
- conditions
- data
- Days
- decision
- departments
- designated
- Devices
- document
- drug
- Drugs
- emergency
- equipment
- equipped
- Ether (ETH)
- events
- evidence
- faster
- Focus
- For
- Force
- Free
- from
- get
- Government
- granted
- Have
- Health
- Hospital
- HTTPS
- import
- in
- Institution
- institutions
- instructions
- International
- issue
- Issued
- IT
- ITS
- jpg
- Legal
- Licensing
- List
- listing
- local
- make
- management
- Manufacturers
- March
- Marketing
- measures
- medical
- medical devices
- Meet
- monitoring
- months
- names
- National
- needed
- news
- of
- on
- Opinion
- overseas
- Personnel
- pilot
- plans
- plato
- Plato Data Intelligence
- PlatoData
- Product
- Products
- professional
- Program
- qualifications
- reaction
- real
- real world
- received
- Registration
- regulatory
- replaced
- responsibility
- responsible
- review
- reviewing
- risks
- roche
- Safety
- same
- serious
- sold
- Source
- special
- Status
- storage
- supply
- support
- suspended
- Systems
- that
- The
- The Source
- therapy
- Through
- to
- Tourism
- trade
- Training
- transportation
- trial
- urgency
- urgent
- Usage
- Water
- webinar
- which
- will
- with
- within
- working
- world
- zephyrnet