NMPA published the finalized document “Guideline on Medical Device Real-world Study Design and Statistical Analysis” on January 15, 2024. The draft version was released on September 28, 2023.
Significance of the Guideline
The guideline will facilitate overseas manufacturers to further explore the Hainan Real-world Data pilot program.
Hainan Boao Pilot Zone allows overseas unapproved medical devices, IVDs, drugs, with clinical urgency status to be used in China. While commercializing and selling their products, manufacturers can collect RWD through RWS in Hainan as local clinical evidence in China to support their national NMPA registration approval. The special program might fasten their China market access to 4 months.
Hainan government released the “Regulations on the Administration of Urgent Use Imported Drugs and Medical Devices in the Boao Lecheng International Medical Tourism Pilot Zone of Hainan Free Trade Port” on March 28, 2023. The document says that It does NOT require local type testing or significant burden of proof as the national approval.
Highlights of the Guideline
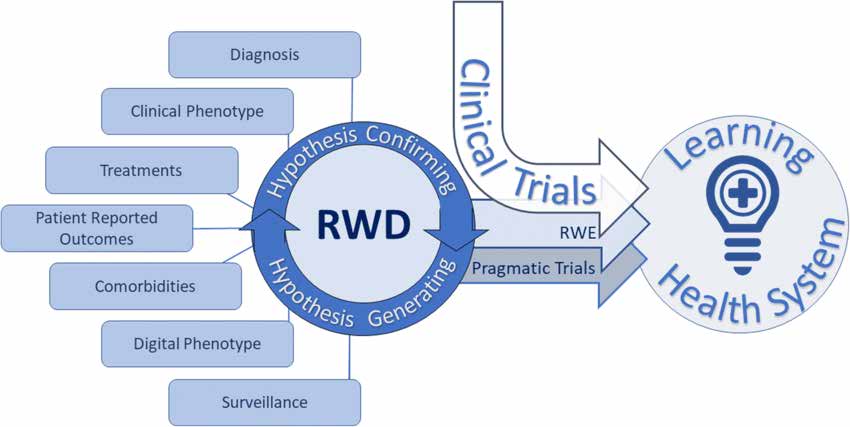
The document on Study Design and Statistical Analysis describes different types of real-world research as following:
Pragmatic Randomized Controlled Trials (pRCTs) are designed to assess treatment outcomes in real healthcare settings. They employ randomization and control groups to evaluate intervention effectiveness. The pRCTs provide high-quality real-world evidence and are suitable for diverse patient populations and clinical scenarios.
Observational Real-World Research includes various study designs:
- Descriptive Study Designs describe patient characteristics or health conditions without inferring causality.
- Cohort Designs track outcomes in different patient groups based on device use, with prospective and retrospective options.
- Case-Control and Derived Designs compare patients with and without device use and include nested case-control and case-cohort studies.
- Real-World Data as External Controls involve using real-world data as a control group, though detailed guidelines are lacking.
The document outlines considerations for designing real-world research protocols:
- Background and Objectives: Define research background, objectives, and safety and effectiveness issues based on existing data.
- Feasibility Assessment: Evaluate the feasibility of conducting real-world research, considering data availability, quality, and confounding variables.
- Select Appropriate Research Design: Choose the design based on objectives, whether experimental or observational.
- Study Flowchart: Create a flowchart detailing the study process, including patient selection, interventions, and examinations.
- Define Study Population: Clearly define inclusion and exclusion criteria for the study population.
- Device Exposure: Assess how patients are exposed to the device, considering potential biases.
- Control Group: Determine suitable control groups for observational studies to balance confounding variables.
- Outcome Measures: Define outcome measures, including their purpose, definition, and measurement methods.
- Data Collection: Develop data collection forms and dictionaries, specifying data sources, quality information, and linkage methods.
- Adjustment for Confounding Variables: Identify confounding variables and include/exclude them with reasons.
- Follow-up Time: Define patient follow-up or observation times to answer research questions adequately.
- Sample Size and Power Calculation: Calculate sample size and statistical power, considering various factors.
It also emphasizes the importance of Quality Control:
- Data Quality: Evaluate data quality in terms of representativeness, completeness, accuracy, and more.
- Bias Risk: Describe measures to control bias risks at various stages, including selection, information, and confounding bias.
- Assessment of Bias: Recognize different bias types and assess their direction and magnitude.
- Ethical Review: Ensure ethical reviews and informed consent comply with regulations.
Comparison of Two Versions
Compared with the draft version, the final document mentions the “Determining Confounding Variables that Need Modification” in the Statistical Analysis section.
In non-randomized real-world research designs, identifying confounding variables is crucial for controlling bias. Three criteria are used to identify them: a variable has a causal relationship with the outcome, is associated with the grouping variable (exposure), and is not an intermediate variable in the causal path. A recommended approach involves a rational variable selection process based on professional knowledge and clinical experience, with collaboration with clinical and statistical experts. For uncertain variables, sensitivity analysis is suggested, providing reasons, and supporting data for inclusion/exclusion. A conservative variable selection approach is advised, avoiding unrelated variables, and considering issues like multicollinearity and interaction effects, visualized through Directed Acyclic Graphs (DAGs).
For an English copy of the guideline, please email info@ChinaMedDvice.com. We charge nominal fees for the translation.
View the latest news on Hainan Real World Data program.
View the communication guideline working with regulatory authorities.
View our recorded webinar on Hainan policies.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://chinameddevice.com/real-world-study-nmpa/
- :has
- :is
- :not
- 15%
- 2023
- 2024
- 28
- a
- access
- accuracy
- acyclic
- adequately
- administration
- advised
- allows
- also
- an
- analysis
- and
- answer
- approach
- appropriate
- approval
- ARE
- AS
- assess
- associated
- At
- Authorities
- availability
- avoiding
- background
- Balance
- based
- BE
- bias
- biases
- burden
- calculate
- CAN
- characteristics
- charge
- China
- Choose
- clearly
- Clinical
- collaboration
- collect
- collection
- COM
- comply
- conditions
- conducting
- consent
- conservative
- considerations
- considering
- control
- controlled
- controlling
- controls
- create
- criteria
- crucial
- data
- data quality
- define
- definition
- Derived
- describe
- describes
- Design
- designed
- designing
- designs
- detailed
- Detailing
- Determine
- develop
- device
- Devices
- different
- directed
- direction
- diverse
- document
- does
- draft
- Drugs
- effectiveness
- effects
- emphasizes
- English
- ensure
- ethical
- evaluate
- evidence
- existing
- experience
- experimental
- experts
- explore
- exposed
- Exposure
- external
- facilitate
- factors
- feasibility
- Fees
- final
- finalized
- following
- For
- forms
- Free
- further
- Government
- graphs
- Group
- Group’s
- guidelines
- Health
- healthcare
- high-quality
- How
- HTTPS
- identify
- identifying
- importance
- in
- include
- includes
- Including
- inclusion
- information
- informed
- interaction
- Intermediate
- International
- intervention
- interventions
- involves
- issues
- IT
- January
- jpg
- knowledge
- lacking
- leader
- like
- local
- Manufacturers
- March
- Market
- measurement
- measures
- medical
- medical device
- medical devices
- mentions
- methods
- might
- months
- more
- National
- Need
- objectives
- observation
- observational
- of
- on
- Options
- or
- our
- Outcome
- outcomes
- outlines
- overseas
- path
- patient
- patients
- pilot
- plato
- Plato Data Intelligence
- PlatoData
- please
- policies
- population
- populations
- potential
- power
- process
- Products
- professional
- Program
- proof
- prospective
- protocols
- provide
- providing
- published
- purpose
- quality
- Questions
- Randomized
- Rational
- real
- real world
- reasons
- recommended
- Registration
- regulations
- regulatory
- relationship
- released
- require
- research
- Reviews
- risks
- Safety
- says
- scenarios
- Section
- selection
- Selling
- Sensitivity
- September
- settings
- significant
- Size
- Sources
- special
- stages
- statistical
- Status
- studies
- Study
- suitable
- support
- Supporting
- terms
- Testing
- that
- The
- their
- Them
- they
- though?
- three
- Through
- times
- to
- Tourism
- track
- trade
- Translation
- treatment
- trials
- two
- type
- types
- Uncertain
- urgency
- urgent
- use
- used
- using
- variable
- various
- version
- was
- we
- whether
- while
- will
- with
- without
- working
- world
- zephyrnet