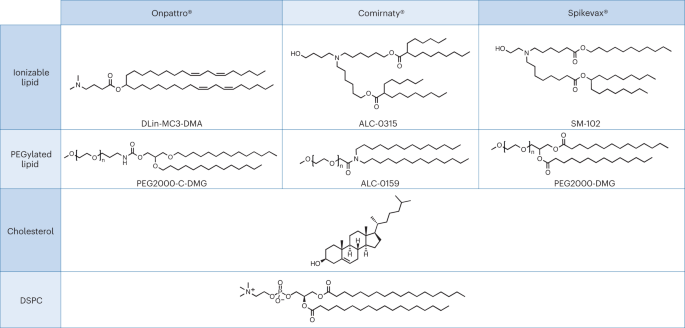
A pertinent case study for the above premise, which also highlights a dissimilarity in regulatory review, can be found in the recent approval of ribonucleic acid (RNA)-based nanomedicines. Closely related LNPs have been used as the delivery system for the following three RNA drugs: Alnylam’s RNAi-based therapy for the treatment of hereditary transthyretin (hATTR) amyloidosis (trade name: Onpattro); the Pfizer–BioNTech COVID-19 vaccine based on mRNA technology (trade name: Comirnaty); and the Moderna COVID-19 vaccine (trade name: Spikevax), also based on mRNA technology. The individual components of the three LNP products are very similar (Fig. 1.)16. In brief, the LNPs are comprised of an ionizable cationic lipid, a PEGylated lipid, cholesterol, and a structural lipid (distearoylphosphatidylcholine; DSPC).
LNPs used in Onpattro, Comirnaty, and Spikevax share several characteristics. Specifically, all three products are made up of a combination of four different lipid types. Two of these lipids, namely cholesterol and DSPC, are identical for all three products. The other two lipids are ionizable lipids with a tertiary amine group and PEGylated-lipids, which are similar for all three products. Overall, the LNPs in the three products share a resemblance in composition and structure.
All three drugs are approved by the FDA and the EMA. Yet despite having very similar LNP compositions, Spikevax’s LNP components were classified differently by the applicant. This classification was accepted by the FDA, and consequently, Spikevax LNP was reviewed differently than the cognate LNPs in the other two products. We compare and contrast the respective regulatory dossiers below. For EMA these details are found in the European Public Assessment Report (EPAR), and for the US FDA, the information is contained in the publicly accessible review and approval documents (FDA approval letters, product labels, Summary Basis for Regulatory Action and Review Memoranda).
Onpattro
According to EMA, the Onpattro drug product is an LNP formed by a mixture of four lipid excipients that encapsulate the double-stranded siRNA (ds-siRNA) patisiran sodium (active substance). Two of the lipids, DLin-MC3-DMA and PEG2000-C-DMG, are considered novel excipients17. The US FDA similarly considers the four lipid components forming the LNP as excipients, with DLin-MC3-DMA and PEG2000-C-DMG also designated as novel18.
Spikevax
In the initial submission of their regulatory dossier, Moderna declared the mRNA and the lipid components as the drug substance19. During EMA’s review of this first version, it was pointed out that only the mRNA should be considered as the active substance. The Spikevax dossier therefore had to be amended to be in line with EU requirements, since EMA regards all four lipid components of the LNP as excipients. Two of these are considered novel, namely, SM-102, an ionisable lipid excipient, and the polyethylene glycol-lipid conjugate, PEG2000-DMG (ref. 19).
In contrast to EMA’s review, FDA accepted Moderna’s classification of PEG2000-DMG and SM-102 as ‘starting materials’ for the drug substance, rather than as excipients20 and the regulatory dossier remained structured accordingly. The full list of excipients does not include PEG2000-DMG and SM-102 (nor the two remaining lipids) and the Chemistry Manufacturing and Controls (CMC) BLA Review Memorandum explicitly states that the mRNA-1273 drug product does not contain any novel excipients. FDA’s summary basis for regulatory action also lists the LNP under the description of the active ingredient21. Juxtaposed to its own ruling in the CMC section, FDA’s toxicology review for Spikevax22 identifies SM-102 and PEG2000-DMG as ‘inactive ingredients’, hence regarding SM-102 and PEG2000-DMG as excipients rather than starting materials for the drug substance.
Comirnaty
Consistent with their review of Spikevax, EMA considers Comirnaty’s structural lipids DSPC and cholesterol and functional lipids ALC-0315 and ALC-0159 as excipients, with the latter two being considered as novel23. In contrast to this, and to its ruling on Spikevax, FDA states that Comirnaty contains four pharmacologically inactive lipid excipients. Namely, DSPC, cholesterol, ALC-0159 and ALC-0315, with the latter two described as novel excipients24. According to FDA’s summary basis for regulatory action, the four lipids forming the Comirnaty LNP have a function of a ‘lipid component’ whereas all other ingredients, also supposedly inactive ingredients, are considered as excipients25.
In short, the FDA reviewed the lipids in Spikevax as part of the drug substance, whereas very similar lipids in Onpattro and Comirnaty were reviewed as excipients. EMA was more consistent in their review, as the lipids in all three LNPs are listed as excipients. We emphasize here that our case study for these three LNPs does not assess the proprietary data provided in the regulatory dossiers, and is limited to publicly available information.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Minting the Future w Adryenn Ashley. Access Here.
- Source: https://www.nature.com/articles/s41565-023-01371-w
- :is
- :not
- $UP
- 1
- 20
- 2017
- 2018
- 2021
- 2022
- 22
- 23
- 24
- a
- above
- accepted
- accessible
- According
- accordingly
- Action
- active
- All
- also
- an
- Anchor
- and
- any
- approval
- approved
- ARE
- AS
- assessment
- available
- based
- basis
- BE
- been
- being
- below
- button
- by
- CAN
- case
- case study
- characteristics
- chemistry
- classification
- classified
- click
- closely
- CMC
- combination
- compare
- components
- Comprised
- Consequently
- considered
- considers
- consistent
- contains
- contrast
- controls
- COVID-19
- data
- debate
- delivery
- described
- description
- designated
- Despite
- details
- different
- documents
- does
- drug
- Drugs
- during
- EMA
- emphasize
- Ether (ETH)
- EU
- Europa
- European
- false
- fda
- Fig
- Figure
- First
- following
- For
- formed
- found
- four
- full
- function
- functional
- Group
- had
- Have
- having
- hence
- here
- highlights
- HTTPS
- identical
- identifies
- image
- in
- inactive
- include
- individual
- information
- initial
- IT
- ITS
- Labels
- Limited
- Line
- LINK
- List
- Listed
- Lists
- made
- manufacturing
- materials
- Memorandum
- mixture
- moderna
- more
- mRNA
- name
- namely
- Nature
- novel
- of
- on
- only
- Other
- our
- out
- overall
- own
- part
- plato
- Plato Data Intelligence
- PlatoData
- Product
- Product Quality
- Products
- proprietary
- provided
- public
- publicly
- quality
- rather
- recent
- regarding
- regards
- regulatory
- related
- remained
- remaining
- report
- Requirements
- respective
- review
- reviewed
- RNA
- ruling
- Section
- several
- Share
- Short
- should
- similar
- Similarly
- since
- Size
- specifically
- Starting
- States
- structural
- structure
- structured
- Study
- submission
- substance
- SUMMARY
- system
- Technology
- tertiary
- than
- that
- The
- the information
- their
- therapy
- therefore
- These
- this
- three
- to
- trade
- treatment
- true
- two
- types
- under
- us
- used
- Vaccine
- version
- very
- View
- vs
- was
- we
- were
- which
- with
- yet
- zephyrnet