NMPA granted innovation approval to Varian’s Proton Therapy System on November 1, 2023.
What’s the Device
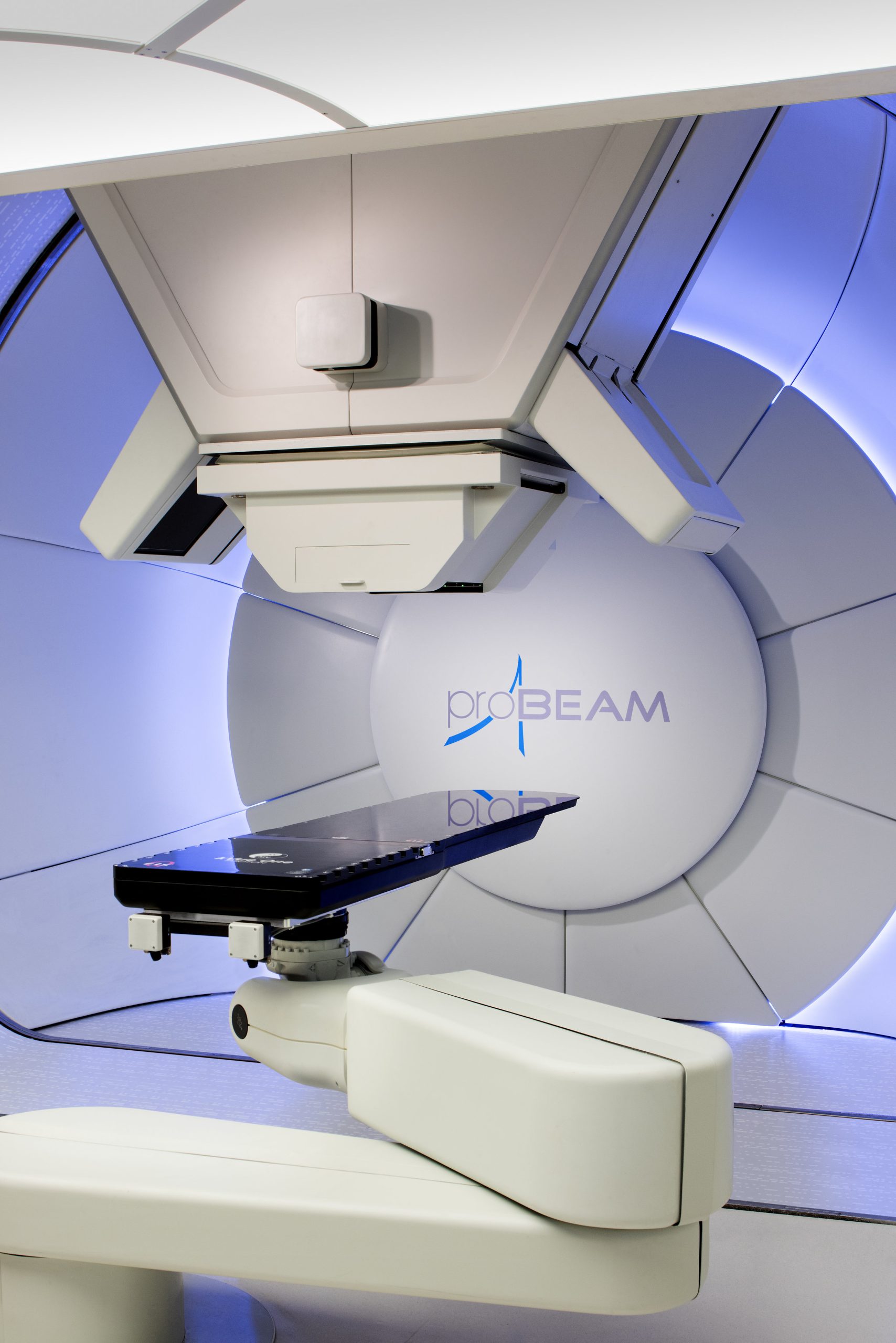
This product consists of an accelerator subsystem and a treatment subsystem. The accelerator subsystem includes the main accelerator system, energy selection system, and beam delivery system, while the treatment subsystem comprises three treatment rooms, including a 360-degree rotational beam therapy system and a treatment planning system. This product provides proton beam radiotherapy and is suitable for treating malignant tumors throughout the body and certain benign conditions. The specific indications are determined by the clinical physician based on the individual case.
In the approval notice, NMPA says that “it is the first approved proton therapy system to utilize superconducting cyclotron accelerator technology and a 360-degree rotational gantry. The mentioned technologies ensure a compact product design and the ability to perform treatments from multiple angles. At the same time, this product can effectively reduce patient treatment time while ensuring treatment effectiveness.”
There have been 233 innovation devices approved since 2014 when the channel was established. To get an English copy of innovation devices catalog, please email info@ChinaMedDevice.com.
We have translated the “Innovation Device Approval Procedure” for you.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://chinameddevice.com/nmpa-proton-therapy-system/
- :is
- 1
- 2014
- 2023
- 360-degree
- a
- ability
- accelerator
- an
- and
- approval
- approved
- ARE
- At
- based
- Beam
- been
- body
- by
- CAN
- case
- catalog
- certain
- Channel
- Clinical
- COM
- compact
- comprises
- conditions
- consists
- delivery
- delivery system
- Design
- determined
- device
- Devices
- effectively
- effectiveness
- energy
- English
- ensure
- ensuring
- established
- First
- For
- from
- Gains
- get
- granted
- Have
- HTTPS
- includes
- Including
- indications
- individual
- Innovation
- jpg
- Main
- mentioned
- multiple
- Notice..
- November
- of
- on
- patient
- perform
- physician
- planning
- plato
- Plato Data Intelligence
- PlatoData
- please
- Product
- product design
- provides
- Radiotherapy
- reduce
- Rooms
- same
- says
- selection
- since
- specific
- suitable
- system
- Technologies
- Technology
- that
- The
- therapy
- this
- three
- throughout
- time
- to
- treating
- treatment
- treatments
- tumors
- utilize
- was
- when
- while
- you
- zephyrnet