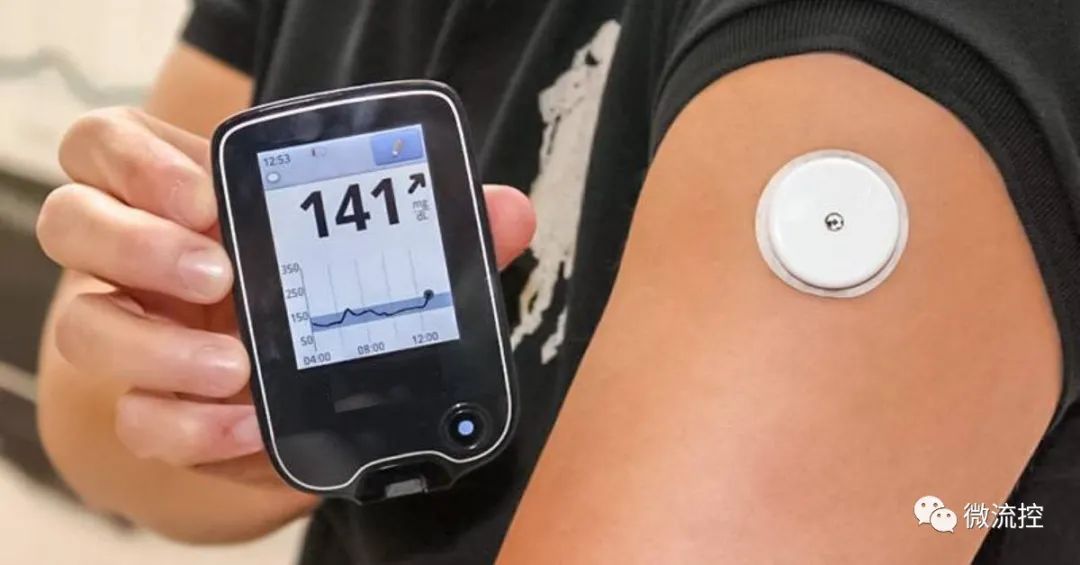
NMPA released the “Guideline on Continuous Glucose Monitoring System” on July 18, 2023. It is the finalized version from the draft issued in September 2022.
Highlights of the Guideline
For an English copy of the 58-page “Guideline on Continuous Glucose Monitoring System”, please email info@ChinaMedDevice.com. We charge nominal fees for the translation.
Application Scope
The guideline applies to invasive CGMS. Products with blood glucose/blood ketone/other detection modules should also meet the relevant guidelines such as the “Guideline on Glucose Meters”.
For CGMS connected to smart mobile terminals, automatic insulin dosing devices, and manually controlled therapeutic instruments, the relevant guidelines should be considered, such as “Guideline on Mobile Medical Devices” and “Guideline on Insulin Pumps”.
The guideline excludes non-invasive and implantable CGMS.
Registration Unit
The CGMS include single-use glucose sensors, transmitters, accessories, etc., and should be registered as a singular registration unit. The sensor is the core component of the system which shall be the basis of registration unit division:
- If the sensor material is different, it should be divided into different registration units.
- When the reaction principle or action mechanism of the sensor is different, it should be divided into different registration units.
- Retrospective and real-time CGMS should be divided into different registration units. A real-time CGM system that includes a retrospective continuous glucose monitoring mode can serve as the same registry unit.
- If the performance indicators of the system are quite different, they should be divided into different registration units.
- If the sensor design is different, and the wearing days are quite different, in principle, it should be divided into different registration units; the sensor design is the same, and the different wearing days can be divided into the same registration unit through software settings.
- When the application scope, core algorithm, and performance indicators of the application program for hospital use and home use in conjunction with the hardware implementation of the product are quite different, they should be divided into different registration units.
- For example, home applications with cloud correction functions and hospital applications with manual correction functions should be divided into different registration units.
- For example, applications for hospital use and home use can be divided into the same registration unit only through the authority management of professionals and non-professionals to distinguish hospital and household applications.
- Applications with blood glucose correction algorithms, applications that input calibration data and transfer to CGMS, should be registered as the same unit as CGMS.
- The mobile application for secondary display of real-time patient data in CGMS should be divided into different registration units from CGMS.
Predicate Comparison Circumstances
The information on the predicate products or previous generation products should be described. The reasons for choosing it as a research and development reference should be explained:
- the list compares and explains the working principle (such as invasiveness, chemical reaction), structural composition, manufacturing materials (including animal-derived materials, pharmaceutical ingredients, biologically active substances, conforming standards, etc.), performance indicators, and functions of the product and the reference product. Similarities and differences in methods (such as implantation, intervention), scope of application, sensor storage conditions (temperature, humidity), operating temperature and sterilization validity period.
- The sensor performance index comparison data should at least include glucose monitoring range, linearity, response time, repeatability, stability, drug interference, temperature response, oxidation reaction, waterproof performance, sensor wearing time (hours), calibration times, alarm function, detection Frequency (minutes), data transmission method (Bluetooth/Near Field Communication), etc.
- In the comparison materials, attention should be paid to highlight the differences between the registered products and the comparison products, as well as the primary basis and performance of the different points and should be described.
Special Research Materials
The research materials should specify evaluation indicators, acceptance criteria, test methods (sample size and formulation basis, test equipment, test steps, etc.), data analysis methods, test results, and test conclusions:
- Provide research data on the control principle of glucose concentration by a continuous glucose sensor glucose limiting membrane. The current responses of the enzyme electrode (without the glucose limiting membrane) and the coated sensor (with the glucose limiting membrane coated on the enzyme electrode) in glucose solution were tested respectively, and the linear correlation coefficient and sensitivity were calculated.
- Provide hydrogen peroxide reverse osmosis potential study materials (if applicable). Provide research data on oxygen response (if applicable).
- Provide research data on the effect of pH change of tissue fluid on sensor performance caused by hydrogen ion accumulation, including linearity, stability, and pH change verification. Linearly observe the corresponding changes of the sensor in the high-concentration glucose system; the stability test should be carried out in the higher-concentration glucose system for the stability test of the service life to observe the performance change of the sensor; the pH value change verification observes the pH in the test system before and after the test.
- Provide research data on whether the sensor has the immunogenic problem caused by foreign protein shedding during the claimed wearing days and can provide the immunogenic safety evaluation data of the marketed products.
- During the manufacturing process, research data on intra-batch and inter-batch differences should be provided, and a research report on ensuring the consistency of sensor performance in the manufacturing process or a research report on product performance consistency through quality inspection methods should be provided. Test reports for linearity, repeatability, response time, stability, and temperature response of three consecutive batches of products should be provided.
- Provide information on sensor calibration or control of calibration-free systems (factory calibration).
- If the product is suitable for household use, research data suitable for household use should be provided with reference to the YY9706.111 standard.
- If applicable, supporting information should be provided that the wireless equipment complies with the relevant regulations of radio management, and research information on the effectiveness of wireless transmission should be provided.
- Products using nanomaterials should provide relevant research materials in accordance with the “Guideline for the Safety and Effectiveness Evaluation of Medical Devices Using Nanomaterials Part 1: System Framework”.
The guideline attaches six annexes:
- Product information appendix
- Standards to comply
- Product Technical Requirement
- Calculation formula for technical indicators
- Calibration-free research data
- Anti-interference
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://chinameddevice.com/nmpa-continuous-glucose-monitoring-system-2/
- :has
- :is
- 1
- 2022
- 2023
- a
- acceptance
- accessories
- accordance
- accumulation
- Action
- active
- After
- alarm
- algorithm
- algorithms
- also
- an
- analysis
- and
- applicable
- Application
- applications
- approved
- ARE
- AS
- At
- attention
- authority
- Automatic
- basis
- BE
- before
- between
- blood
- by
- calculated
- CAN
- carried
- caused
- change
- Changes
- charge
- chemical
- choosing
- claimed
- Cloud
- COM
- Communication
- comparison
- component
- concentration
- conditions
- conjunction
- connected
- consecutive
- considered
- continuous
- control
- controlled
- Core
- Correlation
- correlation coefficient
- Corresponding
- criteria
- Current
- data
- data analysis
- Days
- described
- Design
- Detection
- Development
- Devices
- differences
- different
- Display
- distinguish
- divided
- Division
- dosing
- drug
- during
- effect
- effectiveness
- English
- ensuring
- equipment
- etc
- evaluation
- example
- explained
- Explains
- factory
- Fees
- field
- final
- finalized
- fluid
- For
- foreign
- formula
- formulation
- Frequency
- from
- function
- functions
- generation
- guidelines
- Hardware
- Highlight
- Home
- Hospital
- HOURS
- household
- How
- HTTPS
- hydrogen
- if
- implementation
- in
- include
- includes
- Including
- index
- Indicators
- information
- ingredients
- input
- instruments
- intervention
- into
- Issued
- IT
- jpg
- July
- least
- Life
- List
- management
- manual
- manually
- manufacturing
- material
- materials
- mechanism
- medical
- medical devices
- Meet
- method
- methods
- minutes
- Mobile
- Mode
- Modules
- monitoring
- Nanomaterials
- observe
- Observes
- of
- on
- only
- operating
- or
- Osmosis
- out
- Oxygen
- paid
- part
- patient
- patient data
- performance
- period
- Pharmaceutical
- plato
- Plato Data Intelligence
- PlatoData
- please
- points
- potential
- previous
- primary
- principle
- Problem
- process
- Product
- Products
- professionals
- Program
- Protein
- provide
- provided
- quality
- Radio
- range
- reaction
- real-time
- reasons
- registered
- Registration
- registry
- regulations
- released
- relevant
- report
- Reports
- research
- research and development
- respectively
- response
- responses
- Results
- Reveals
- reverse
- Safety
- same
- scope
- secondary
- Sensitivity
- sensors
- September
- serve
- service
- settings
- should
- similarities
- singular
- SIX
- Size
- smart
- Software
- solution
- Stability
- standard
- standards
- Steps
- storage
- structural
- Study
- such
- suitable
- Supporting
- system
- Systems
- Technical
- test
- tested
- that
- The
- Therapeutic
- they
- three
- Through
- time
- times
- tissue
- to
- transfer
- Translation
- transmitters
- unit
- units
- use
- using
- value
- Verification
- version
- we
- WELL
- were
- whether
- which
- wireless
- with
- without
- working
- zephyrnet