In this article, we will go through the requirements for Trend Reporting, that are summarized within Article 88 of the EU MDR ,and Article 83 of the EU IVDR. Trend reporting is a process belonging to post-market surveillance, a topic that we have been extensively discussing in other posts, dealing with topics such as periodic safety update report, post-market surveillance plan, vigilance reporting and much more.
The purpose of trend reporting is to monitor, over time, the number of incidents not classified as serious incidents, in order to determine if the benefit-risk analysis of the device has changed.
The concept of Trend Reporting is strictly related with post-market risk management. The final goal is to keep under control non-serious incidents and ensure that risk management documentation is kept up to date while maintaining a positive benefit/risk ratio.
Guidelines for Trend Reporting Process
The concept of Trend Reporting is not new, and it was already present in different guidelines and regulations.
In fact, the topics related to trend reporting are well explained in the MEDDEV 2.12, focused on post-market surveillance for EU. This MEDDEV guideline, although it has been developed for the previous medical device directive, it is still applicable, in absence of a more recent guideline on post-market surveillance requirements for MDR.
Moreover, there are as well two other guidelines developed by the Global Harmonization Task Force; the first one is related adverse event reporting and the second on focused on trend reporting of adverse events. Both these guidelines have specific sections related to trend reporting, providing insightful guidelines on the practical implementation of the trend reporting process.
In general, trend reports are related to three different categories of events:
- All the Non-serious incidents / events
- Adverse events currently exempted from reporting
- Adverse events scheduled for periodic reporting.
However, we will focus on trend reports related to non-serious incidents, that is the focus of the EU MDR and IVDR.
Trend Reporting and EU MDR / IVDR
As mentioned before, the requirements associated to trend reporting are defined within Article 88 of the EU MDR.
This article basically requires that trend reporting shall be activated when there is a statistically significant increase in the frequency or severity of incidents, that are not serious incidents, or that are expected undesirable side- effects that could have a significant impact on the benefit-risk analysis.
As it can be understood, in order to properly implement an efficient trend reporting process, that addresses the requirements of the EU regulations, it is necessary to have a robust statistic-based approach that helps to identify trends that would need to be reported.
Obviously, there might be many different processes that can be implemented in order to build an efficient trend reporting process. In the next slides, we are going to propose an example of approach that can be used to identify reportable trends.
Practical Approaches for Trend Reporting
One of the possible approaches that can be used to identify trends of non-serious incidents is based on the so-called Product Problem Rate.
Given the number of medical devices (d) in the market over a specific timeframe (t), and considering the number of non-serious events over the same timeframe (t), the Product Problem rate is defined as the ratio between the number of events, and the number of devices on the market, in relation to a specific timeframe.
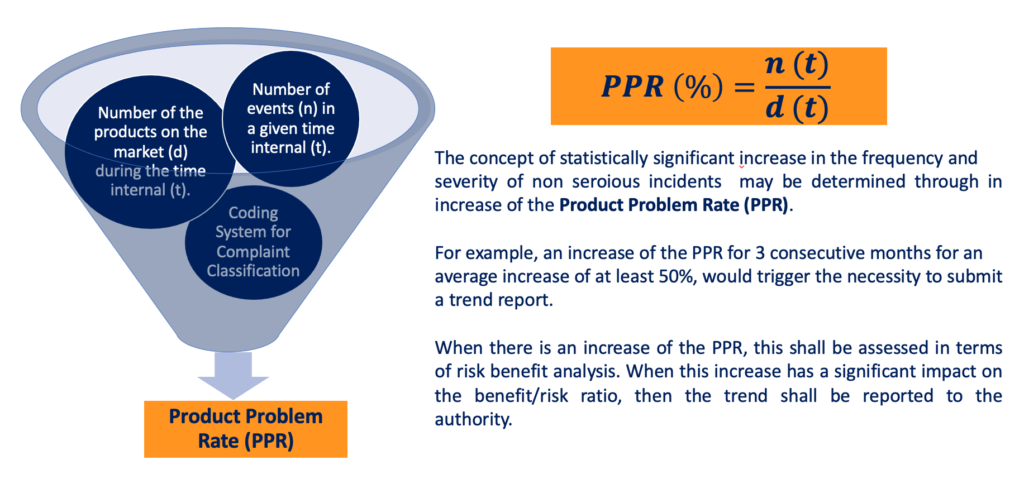
The concept of statistically significant increase in the frequency and severity of non serious incidents, may be determined through an increase of the Product Problem Rate.
For example, an increase of the PPR for 3 consecutive months, for an average increase of at least 50%, could potentially be an indication of an adverse trend that would need to be reported.
Moreover, when there is an increase of the PPR, this shall be assessed in terms of risk benefit analysis, in the context of the so-called post-market risk management, that we will discuss in the last section of this course.
Subscribe to QualityMedDev Newsletter
QualityMedDev is an online platform focused on Quality & Regulatory topics for medical device business; Follow us on LinkedIn and Twitter to stay up to date with most important news on the Regulatory field.
QualityMedDev is one of the largest online platform supporting medical device business for regulatory compliance topics. We provide regulatory consulting services over a broad range of topics, from EU MDR & IVDR to ISO 13485, including risk management, biocompatibility, usability and software verification and validation and, in general, support in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy provides the possibility to follow online and self-paced training courses focused on regulatory compliance topics for medical device. These training courses, developed in collaboration with highly skilled professionals in the medical device sector, allows you to exponentially increase your competencies over a broad range of quality and regulatory topics for medical device business operations.

Do not hesitate to subscribe to our Newsletter!
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.qualitymeddev.com/2022/10/12/trend-reporting/
- a
- Academy
- According
- addresses
- adverse
- allows
- already
- Although
- analysis
- and
- applicable
- approach
- approaches
- article
- assessed
- associated
- average
- based
- Basically
- before
- benefit
- between
- broad
- build
- business
- categories
- classified
- collaboration
- compliance
- concept
- consecutive
- considering
- consulting
- context
- control
- could
- course
- courses
- Currently
- Date
- dealing
- Determine
- determined
- developed
- device
- Devices
- different
- discuss
- discussing
- documentation
- effects
- efficient
- ensure
- Ether (ETH)
- EU
- Event
- events
- example
- expected
- explained
- exponentially
- field
- final
- First
- Focus
- focused
- follow
- Force
- Frequency
- from
- General
- Global
- Go
- goal
- going
- guidelines
- helps
- highly
- HTTPS
- identify
- Impact
- implement
- implementation
- implemented
- important
- in
- In other
- Including
- Increase
- IT
- Keep
- largest
- Last
- mailchimp
- management
- many
- Market
- max-width
- MDR
- medical
- medical device
- mentioned
- might
- Monitor
- months
- more
- most
- Navigation
- necessary
- Need
- New
- news
- next
- number
- ONE
- online
- Operations
- order
- Other
- periodic
- platform
- plato
- Plato Data Intelligence
- PlatoData
- plugin
- positive
- possibility
- possible
- Posts
- potentially
- Practical
- present
- previous
- Problem
- process
- processes
- Product
- professionals
- properly
- propose
- provide
- provides
- providing
- purpose
- quality
- range
- Rate
- ratio
- recent
- regulations
- regulatory
- Regulatory Compliance
- related
- relation
- Reported
- Reporting
- Reports
- Requirements
- requires
- Risk
- risk management
- robust
- Safety
- same
- scheduled
- Second
- Section
- sections
- sector
- serious
- significant
- skilled
- Slides
- Software
- specific
- stay
- Still
- subscribe
- such
- support
- Supporting
- surveillance
- Task
- task force
- Technical
- terms
- The
- three
- Through
- time
- timeframe
- to
- topic
- Topics
- Training
- Trend
- Trends
- under
- understood
- Update
- URL
- us
- usability
- validation
- Verification
- while
- will
- within
- WordPress
- WordPress plugin
- would
- Your
- zephyrnet