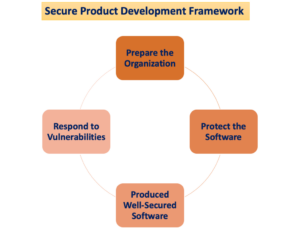
The IVDR – In vitro Diagnostic Regulation 2017/746 – will enter into force in 2022 but it is very important to discuss since now the changes that this new regulation will trigger in the In Vitro Diagnostic sector. The introduction of the IVDR, along with the sister regulation on medical device (MDR 2017/745), is definitely the major changes in the last 20 years in the medical device and in vitro diagnostic business.
It is widely recognized that the IVDR 2017/746 will bring great and significant changes to IVD business. The changes that will be introduced are across a substantial number of topics, from the classification to the labelling, from post-market surveillance activities to performance evaluation for in vitro diagnostic devices.
We will provide an overview of the main changes, giving as well guidance and suggestions on available documentation that could help in the implementation of the specific changes.
IVD Classification
We have been already discussing about classification of in vitro diagnostic devices according to the IVDR 2017/746. In short, there are four classes of devices, named from A to D depending on the associated risk (Class A for low-risk devices, Class D for high-risk devices). About the 90% of the IVDs, for which “self-certification” is currently possible based on the IVD directive, shall be reclassified according to the IVDR. This reclassification will require the involvement of a Notified Body, that will review the technical documentation and the QMS requirements, in a more stringent and rigorous control context.
Performance Evaluation for IVDR 2017/746
Performance evaluation for in vitro diagnostic device has also been already discussed in QualityMedDev blog, specifically when we have been discussing the ISO 20916:2019 – In vitro diagnostic medical devices — Clinical performance studies using specimens from human subjects — Good study practice.
Performance evaluation for IVD devices is performed for different reasons, however the main one is to avoid false positive and false negative. The IVDR 2017/746 requirements related to performance evaluation are basically the following:
- Having an SOP for performance evaluation established and implemented
- Having a Performance Evaluation Plan that shall address all the requirements listed in the annex XIII of the IVDR 2017/746. We will talk in a different post about performance evaluation plan and report for in vitro diagnostic devices.

A separated mention shall be given to the clinical evaluation and performance evaluation for medical device and in vitro diagnostic devices containing software. The Medical Device Coordination Group (MDCG) published a specific guideline to address this topic (MDCG 2020-01 : Guidance on Clinical Evaluation (MDR) / Performance Evaluation (IVDR) of Medical Device Software).
Clinical evaluation (MDR) / Performance Evaluation (IVDR) is an iterative process which goes through the entire life cycle of the device. This process can be summarized in different steps, which are highlighted below:
- Establishing and maintaining a clinical evaluation plan (MDR) / performance evaluation plan (IVDR), which basically contains the definition of the criteria used to generate clinical evidence.
- Identification of the relevant data related to performance and safety of the device.
- Appraisal of the relevant data in terms of quality and its contribution to the Clinical Evaluation (MDR) /Performance Evaluation (IVDR)
- Document the assessment of data and the clinical evidence derived from this assessment in the Clinical Evaluation (MDR) / Performance Evaluation Report (IVDR).
- Update Clinical Evaluation (MDR) / Performance Evaluation (IVDR) with post-market surveillance data gathered through the Post-Market Clinical Follow-up and Post-market Performance Follow-up.

Traceability of the IVD Devices
Significant changes shave also be introduced from the traceability point of view. We have been extensively discuss about the Unique Device Identification and the implementation of UDI requirements on medical device. The IVDR 2017/746 requires manufacturers of all classes to implement a UDI system for enhanced traceability. Moreover, data will be recorded in the Eudamed system that will replace national registries that have been operated under the IVDD.
Label and Instructions for Use
Labelling and instructions for use will be substantially impacted by the new IVDR 2017/746. Regarding the labelling, as mentioned in the section before, the introduction of the UDI to enhance traceability will impart the necessity to include UDI system in the IVD labels. Moreover, given the change in the IVD classification and the increase of notified body involvement in the CE marking process, many IVD devices will have now a notified body number under the CE marking symbols. There are as well new symbols which will be introduced in the new ISO 15223.
Changes will also need to be performed in the Instructions for Use (IFU). It is important to mention that the new approach defined in the IVDR 2017/746 might trigger a change of the intended purpose of the device. The change of intended purpose might have a huge impact on the registration of the device in different countries, as this might be consider a completely different device.
Responsible Person
Manufacturers shall appoint at least one Responsible Person for Regulatory Compliance, having a well-defined role, tasks and minimum requirements. The requirements associated to the PRRC have been already discussed in QualityMedDev blog.
The new General Safety and Performance Requirements according to IVDR 2017/746
The General Safety and Performance Requirements (SPR) will replace the Essential Requirements (ER) embedded with the previous IVD directive. The SPR are much more comprehensive than the former ER and also bring new or amended requirements, e.g. on labeling. In addition, manufacturers are required to complete the corresponding checklist in much more detail, to properly explain how each specific applicable GSPR is covered. The General Safety and Performance Requirements for IVDR 2017/746 are divided intro three main groups:
- GSPRs 1 to 8 are found in Chapter One of Annex I, General Requirements. These GSPRs apply to all IVDs.
- Requirements Regarding Performance, Design and Manufacture are within GSPRs 10 to 19, mentioned in Chapter II of the IVDR 2017/746; in this case, each GSPR must be considered for its applicability.
- Chapter 3, Requirements Regarding Information Supplied With the Device, is dedicated to one overarching requirement applicable to labels and instructions for use. Within this one GSPR, there are many sub-requirements.
QualityMedDev Newsletter
QualityMedDev is an online platform that provides extensive support to medical device manufacturers and consultancy companies in the field of regulatory compliance. We publish blog posts on quality management system and regulatory-related topics and provide extensive documentation ready to be downloaded to support the implementation and maintenance quality system or product-related certifications. QualityMedDev provides consulting service for quality and regulatory affairs topic for medical device manufacturers, do not hesitate to find out more on our services in the dedicated page of the website. We support the construction of brand new quality system and/or preparation of SW related technical documentation.
We publish as well a periodic newsletter aimed at sharing information on the new articles or documents which have been made available through QualityMedDev website.
If you would like to stay updated with the last news and analysis from the regulatory world for medical device sector, do subscribe to our newsletter by filling the form below.
Source: https://www.qualitymeddev.com/2021/06/05/ivdr-2017-746/
- activities
- All
- analysis
- articles
- Blog
- Blog Posts
- body
- business
- change
- classification
- Companies
- compliance
- construction
- consulting
- countries
- data
- Design
- detail
- Devices
- documents
- form
- General
- Giving
- good
- great
- Group
- Highlighted
- How
- HTTPS
- huge
- Identification
- Impact
- Increase
- information
- IT
- labeling
- Labels
- major
- management
- medical
- medical device
- medical devices
- Navigation
- news
- Newsletter
- online
- performance
- platform
- plugin
- Point of View
- Posts
- publish
- quality
- reasons
- Registration
- Regulation
- Regulatory Compliance
- report
- Requirements
- review
- Risk
- Safety
- Services
- Short
- Software
- stay
- studies
- Study
- support
- surveillance
- system
- Technical
- Topics
- Traceability
- View
- Website
- within
- WordPress
- world
- years