A new version of ISO 17511 has been recently published and in this article we will dig into the requirements of this ISO standard, focused on ensuring metrological traceability for In-vitro Diagnostic Medical Devices. We have been already discussing about IVD devices, particularly in relation to ISO 15189, ISO 23640 related to the stability of the IVD devices, performance evaluation plan, and EU Reference laboratories according to IVDR 2017/746.
Before entering into the detailed requirements of the standard, it is important to define what does exactly mean metrological traceability. According to the International vocabulary of metrology traceability is “a property of a measurement result that can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty”.
It is also deem necessary to fully understand the concept of calibration. In the traceability context, Calibration means using the value of the previous reference material in the traceability hierarchy to assign value through measurement to the next calibrator in the hierarchy. Usually this is typically done by purchasing a certified reference material at two or more appropriate concentrations, which can be used for calibrating a linear measurement equation used for measurement.
Traceability Hierarchies According to ISO 17511
ISO 17511 defines six calibration hierarchies and value transfer models, depending on the availablity of the traceability to SI.
Calibration in the traceability hierarchy is related to the possibility to use the value of the previous reference material in the traceability hierarchy to assign value through measurement to the next calibrator in the hierarchy.
The six material tithing the calibration hierarchies are:
- Certified primary reference material – ISO 15194 conforming;
- Primary calibrator – prepared as solution of m. 1 in suitable solvent;
- Secondary, commutable certified reference material conforming to ISO 15193. Matrix is pooled human plasma;
- Manufacturers working calibration;
- Calibrator for the end-user measurement device;
- Human sample with result
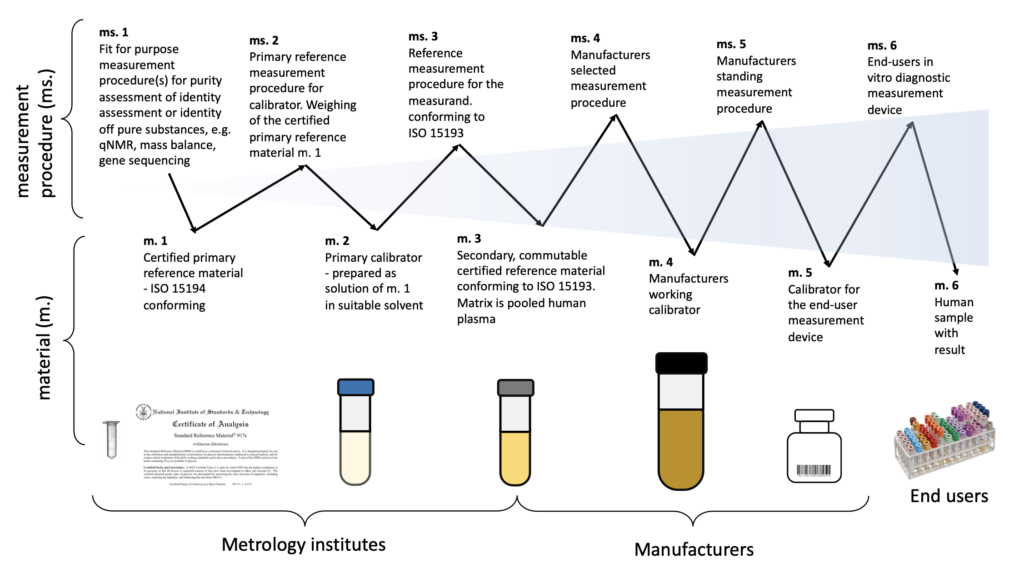
Certified reference materials are self-evident at the top/beginning of the traceability hierarchy and end-user calibrators further on in the hierarchy.
The technical documentation of the calibration hierarchy needs to include a figure or other illustration describing the linkage from the ultimate results using the human samples examined with the specified measuring system up to the highest available metrological reference.
For each step in the calibration hierarchy, the quantity being measured in the relevant reference material or human samples, in the case of the final measurements with a measuring system, must be identified, and the relationship between the measured quantity (or quantities) and the measurand must be established.
prerequisites:
- Traceability to SI
- Availability of certified reference materials
- Availability reference measurement procedures
- Availability of harmonization protocols
The primary reference materials are usually highly purified. They contain a physiochemically well-defined analyte, evaluated for compositional stability integrity, and accompanied by a certificate (t – a certified reference material).
A primary calibrator shall be prepared from a primary reference material and value-assigned using a primary reference measurement procedure.
The value to a secondary calibrator or secondary reference material must be given through an appropriate reference measurement procedure for the measurand.
Subscribe to QualityMedDev Newsletter
QualityMedDev is an online platform focused on Quality & Regulatory topics for medical device business; Follow us on LinkedIn and Twitter to stay up to date with most important news on the Regulatory field.
QualityMedDev is one of the largest online platform supporting medical device business for regulatory compliance topics. We provide regulatory consulting services over a broad range of topics, from EU MDR & IVDR to ISO 13485, including risk management, biocompatibility, usability and software verification and validation and, in general, support in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy provides the possibility to follow online and self-paced training courses focused on regulatory compliance topics for medical device. These training courses, developed in collaboration with highly skilled professionals in the medical device sector, allows you to exponentially increase your competencies over a broad range of quality and regulatory topics for medical device business operations.

Do not hesitate to subscribe to our Newsletter!
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.qualitymeddev.com/2022/12/01/iso-17511/
- 1
- a
- About
- Academy
- According
- allows
- already
- and
- appropriate
- article
- available
- being
- between
- broad
- business
- case
- certificate
- Certified
- chain
- collaboration
- compliance
- concept
- consulting
- context
- contributing
- courses
- Date
- Defines
- Depending
- detailed
- developed
- device
- Devices
- DIG
- discussing
- documentation
- each
- ensuring
- established
- Ether (ETH)
- evaluated
- evaluation
- exactly
- exponentially
- field
- final
- focused
- follow
- from
- fully
- further
- General
- given
- hierarchy
- highest
- highly
- HTTPS
- human
- identified
- important
- in
- include
- Including
- Increase
- integrity
- International
- ISO
- IT
- largest
- mailchimp
- management
- material
- materials
- Matrix
- max-width
- MDR
- measurements
- measuring
- medical
- medical device
- Metrology
- models
- more
- most
- Navigation
- necessary
- needs
- New
- news
- next
- ONE
- online
- Operations
- Other
- particularly
- Plasma
- platform
- plato
- Plato Data Intelligence
- PlatoData
- plugin
- possibility
- Posts
- prepared
- previous
- primary
- professionals
- provide
- provides
- published
- purchasing
- quality
- quantity
- range
- recently
- regulatory
- Regulatory Compliance
- related
- relation
- relationship
- relevant
- Requirements
- Results
- Risk
- risk management
- secondary
- sector
- SIX
- skilled
- Software
- solution
- specified
- Stability
- standard
- stay
- Step
- subscribe
- suitable
- support
- Supporting
- system
- Technical
- The
- Through
- to
- Topics
- Traceability
- Training
- transfer
- typically
- ultimate
- understand
- URL
- us
- usability
- use
- usually
- validation
- value
- value transfer
- Verification
- version
- well-defined
- What
- which
- will
- WordPress
- WordPress plugin
- working
- Your
- zephyrnet