Home > Press > A non-covalent bonding experience: Scientists discover new structures for unique hybrid materials by altering their chemical bonds
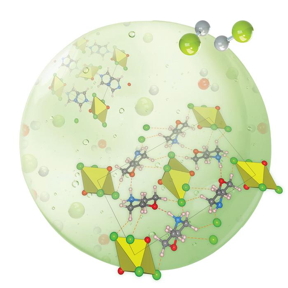 |
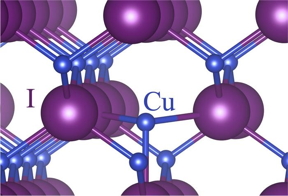
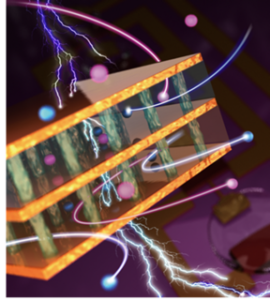
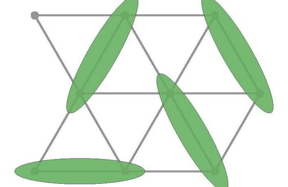
| An artist’s representation of some of the new structures Rajapakha and the team have designed. CREDIT Brookhaven National Laboratory/Angewandte Chemie |
Abstract:
Putting a suite of new materials synthesis and characterization methods to the test, a team of scientists from the University of Iowa and the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory has developed 14 organic-inorganic hybrid materials, seven of which are entirely new. These uranium-based materials, as well as the detailed report of their bonding mechanisms, will help advance clean energy solutions, including safe nuclear energy. The work, currently published online, was recognized as both a Very Important Paper and a Hot Topic: Crystal Engineering in Angewandte Chemie, International Edition. It will appear in July’s print issue.
A non-covalent bonding experience: Scientists discover new structures for unique hybrid materials by altering their chemical bonds
Upton, NY | Posted on July 21st, 2023
While it’s important to understand what a structure is made from, it is just as important to understand what holds it together. Scientists and students from the University of Iowa, with the help of Sara E. Mason, a group leader in theory and computation at the Center for Functional Nanomaterials, a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory, and adjunct associate professor at the University of Iowa, embarked on a quest to understand and manipulate the bonds that support the structure of uranyl tetrahalide, a uranium compound.
“This study was beyond collaborative,” said study co-author Sara E. Mason. “On the synthesis side, we discovered entirely new crystal structures, which is really cool on its own. On top of that, we saw some interesting thermochemistry, the chemical energy stored in the bonds of those structures. Then there was the modeling of these structures. We could have kind of ended our study there, but Harindu Rajapaksha, the student driving this research, really wanted to push it further and use the thermochemistry and the modeling to understand these systems at a level that hasn’t been possible before.”
The layers of work the team contributed resulted in a comprehensive quest to understand and manipulate the bonds that support the structure of uranyl tetrahalide, an important uranium compound. The theoretical and experimental research provided insight into the way hydrogen molecules form bonds that can stabilize these complex molecular structures, paving the way for scientists to alter them for many applications.
Uranyl Tetrahalide—the Remix
When designing hybrid materials to study, why look at uranium? For this team, the answer is both practical and personal.
“In order to effectively manage nuclear waste, we need a better understanding of issues like material separations and recycling,” said Tori Forbes, a professor and director of the Materials Analysis, Testing, and Fabrication Facility at the University of Iowa. “We need to know how uranium behaves in solids and in water, so we are probing the most basic chemistry of uranium to acquire knowledge that can be used for advanced technologies and strategies to improve the back end of the nuclear fuel cycle.”
Looking towards a future with clean energy deployed at scale, uranium is a material that has piqued a lot of scientific interest. Uranium makes up a vast majority of the byproducts from nuclear energy, which is a zero-carbon-emission energy source. Understanding the chemistry of uranium and related systems is integral for implementing nuclear energy safely and effectively. That isn’t the only intriguing aspect of this element though. Some researchers enjoy the challenge of working on such complex structures.
“I’m a chemist by training,” said Mason, “so I’m fascinated by what’s really deep in the periodic table, like uranium. The deeper you go, the more electrons you have, and the more electrons you have, the weirder, more exotic, and more exciting the electronic structure and bonding is. There’s this ‘final frontier’ of the periodic table aspect to it. These are never-before-characterized structures. These are brand new! From a pure, chemical curiosity, this is all really cool.”
This work also built on the foundation of uranyl hybrid materials research that the team published in Inorganic Chemistry in 2022. Both studies used density-functional theory, a computational modelling method that uses quantum mechanics to predict materials’ electronic structure—the way electrons move in certain materials—alongside complementary methods to characterize these structures. In larger molecules, the atomic structure of a chemical system gets more complex, and more electrons are available to interact. Those interactions can make certain calculations difficult, which is why scientists rely on a few different methods to investigate the structure and properties of these systems. By building on the foundations of their previous work, the team now had enough structures to compare the theoretical work to the experiment, which limited them in the past.
Making Connections
LEGO® bricks will snap together and form a strong bond until they are pried apart. Their precisely molded plastic studs and recesses were designed to always work in the same way with all kinds of structures, opening a world of possibilities with each configuration. Molecules have a number of systems to bond atoms together. Some are melded to each other like glue, some click together like LEGO® bricks.
Non-covalent hydrogen bonds can be thought of like an electrostatic force. There is a bond donor, like the studs on the top of a LEGO® brick, that interacts with a bond acceptor, or the back of the brick where the studs fit snugly. These bonds can occur both intra- and intermolecularly, as well as between separate molecules or within the same molecule structure, which allows for all kinds of interesting molecular geometries to arise. The strength of these bonds and the energy held within the bonds change based on the structures they’re in. Understanding the properties of these variations can allow scientists to get creative and discover how to take apart and rebuild useful materials in unexpected ways.
Forbes found that these bonds were more interesting than they appeared on the surface. She explained that “non-covalent interactions (NCIs) like this are often the bonds that get overlooked because they are considered weak. However, when you combine them into a larger network, then it is the sum of these interactions that can have huge impacts on the chemistry. This is more a systems-level approach to understand the chemistry holistically. These types of network systems are incredibly important to the stability of materials and the overall behavior of uranium in water.”
“NCIs are significant in several applications, including drug development and to nuclear waste reprocessing,” explained Rajapaksha. “Our goal was to create a methodology for quantitatively characterizing the NCI network in a well-studied uranyl tetrahalide model system and describe how NCIs affect two crucial uranyl solid phase properties: vibrational and formation enthalpies?a direct indicator of a species’ stability. These properties are significant because vibrational spectroscopy, a method of identifying molecules by the way they absorb light, is a frequently utilized technique for specialized methods that can identify uranyl species.”
Enthalpy is the measurement of the internal energy and pressure energy of a thermodynamic system, which determines the strength of the bonds. When broken, the energy stored in these bonds is released as heat, which can be measured through a process called calorimetry. In this process, a tool called a calorimeter measures the change in temperature that occurs when that heat is transferred out. If that word looks familiar, it’s because calorimetry determines how many calories are in food. Instead of burning materials, however, the team used acid to create a chemical reaction that broke the bonds and gave off heat. Getting the computational modeling to agree with the experimental data, however, took a bit of work.
“Rajapaksha got that to work out really nicely,” said Mason. “He had this high-quality agreement between the model thermochemistry and the measured thermochemistry. This is important because it means that we can rely on his measurements. Even if it’s a system that hasn’t been synthesized yet, he can model it correctly. He can trust those predictions. If we have a reliable way of calculating the thermochemistry, then we can spot trends and gain a new, more complete physical understanding of the bond, chemically speaking, which can allow us to tune and control these interactions.”
The Shape of Things to Come
While the team has learned some interesting things about uranyl tetrahalide systems, they say the most important finding is the cooperative methodology they have developed to characterize these materials. There are other complex chemical structures that the same principles can be applied to, and their applications could have world changing impacts.
“We are really thrilled about our findings,” said Rajapaksha, “and we intend to expand this work in the future to include less-explored systems, such as neptunyl. Neptunium 237, a pollutant, is a long-lived isotope that contributes to the radioactivity of spent nuclear fuels. Basic knowledge in this field would be extremely valuable to basic sciences and nuclear waste management. We have, so far, obtained pretty intriguing results by applying our methodology to the neptunyl system, which we aim to publish soon.”
This research is funded by the Department of Energy, Basic Energy Sciences program under DE-SC0021420 and the Theory and Computation facility of the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. Computational resources (High Performance Computing access) were provided in part by the University of Iowa. All calorimetry measurements were performed at the University of Notre Dame. Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science.
####
About DOE/Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on Instagram, LinkedIn, Twitter, and Facebook.
For more information, please click here
Contacts:
Denise Yazak
DOE/Brookhaven National Laboratory
Office: (631)344-6371
Copyright © DOE/Brookhaven National Laboratory
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
Chemistry
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
News and information
![]() The present and future of computing get a boost from new research July 21st, 2023
The present and future of computing get a boost from new research July 21st, 2023
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
![]() Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Laboratories
Govt.-Legislation/Regulation/Funding/Policy
![]() The present and future of computing get a boost from new research July 21st, 2023
The present and future of computing get a boost from new research July 21st, 2023
Possible Futures
![]() Two types of ultrafast mode-locking operations generation from an Er-doped fiber laser based on germanene nanosheets July 21st, 2023
Two types of ultrafast mode-locking operations generation from an Er-doped fiber laser based on germanene nanosheets July 21st, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
![]() Researchers put a new twist on graphite July 21st, 2023
Researchers put a new twist on graphite July 21st, 2023
Discoveries
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
![]() Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
![]() Researchers put a new twist on graphite July 21st, 2023
Researchers put a new twist on graphite July 21st, 2023
Materials/Metamaterials/Magnetoresistance
![]() Two types of ultrafast mode-locking operations generation from an Er-doped fiber laser based on germanene nanosheets July 21st, 2023
Two types of ultrafast mode-locking operations generation from an Er-doped fiber laser based on germanene nanosheets July 21st, 2023
![]() Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
![]() Researchers put a new twist on graphite July 21st, 2023
Researchers put a new twist on graphite July 21st, 2023
![]() Discovering features of band topology in amorphous thin films June 30th, 2023
Discovering features of band topology in amorphous thin films June 30th, 2023
Announcements
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
![]() Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Understanding the diverse industrial applications of materials science: Materials Science A Field of Diverse Industrial Applications July 21st, 2023
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
![]() New single-photon Raman lidar can monitor for underwater oil leaks: System could be used aboard underwater vehicles for many applications June 30th, 2023
New single-photon Raman lidar can monitor for underwater oil leaks: System could be used aboard underwater vehicles for many applications June 30th, 2023
![]() When all details matter — Heat transport in energy materials June 9th, 2023
When all details matter — Heat transport in energy materials June 9th, 2023
Energy
![]() Graphene-based Carbocatalysts: Synthesis, Properties, and Applications—Beyond Boundaries June 9th, 2023
Graphene-based Carbocatalysts: Synthesis, Properties, and Applications—Beyond Boundaries June 9th, 2023
![]() When all details matter — Heat transport in energy materials June 9th, 2023
When all details matter — Heat transport in energy materials June 9th, 2023
![]() Researchers at Purdue discover superconductive images are actually 3D and disorder-driven fractals May 12th, 2023
Researchers at Purdue discover superconductive images are actually 3D and disorder-driven fractals May 12th, 2023
![]() Channeling mechanical energy in a preferred direction April 14th, 2023
Channeling mechanical energy in a preferred direction April 14th, 2023
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: http://www.nanotech-now.com/news.cgi?story_id=57368
- :has
- :is
- :not
- :where
- $UP
- 10
- 14
- 2022
- 21st
- 26
- 30th
- 3d
- 7th
- 9th
- a
- About
- access
- accuracy
- acquire
- actually
- address
- advance
- advanced
- affect
- Agreement
- aim
- All
- allow
- allows
- also
- always
- an
- analysis
- and
- answer
- apart
- appear
- appeared
- applications
- applied
- Applying
- approach
- April
- ARE
- arise
- artist
- AS
- aspect
- Associate
- At
- available
- Baby
- back
- Bacteria
- BAND
- based
- basic
- BE
- because
- been
- before
- Better
- between
- Beyond
- Bit
- bond
- Bonds
- boost
- both
- brand
- Broke
- Broken
- Building
- built
- burn
- burning
- but
- by
- calculating
- called
- CAN
- Catalyst
- Center
- certain
- CGI
- challenge
- challenges
- change
- changing
- characterize
- chemical
- chemistry
- clean energy
- click
- Co-Author
- collaborative
- COM
- combine
- comment
- compare
- complementary
- complete
- complex
- Compound
- comprehensive
- computation
- computing
- Configuration
- considered
- Containers
- content
- contract
- contributed
- contributes
- control
- Cool
- cooperative
- could
- create
- Creative
- credit
- crucial
- Crystal
- curiosity
- Currently
- Cut
- cycle
- dance
- data
- deep
- deeper
- demonstrate
- Department
- Department of Energy
- Department Of Energy (DOE)
- deployed
- describe
- designed
- designing
- detailed
- details
- determines
- developed
- Development
- different
- difficult
- direct
- Director
- discover
- discovered
- diverse
- DOE
- dramatically
- driving
- drug
- drug development
- dynamics
- e
- each
- edition
- effectively
- Electronic
- electrons
- element
- enable
- end
- energy
- Energy Solutions
- Engineering
- Engines
- enjoy
- enough
- entirely
- Ether (ETH)
- Even
- exciting
- Exotic
- Expand
- experience
- experiment
- experiments
- explained
- Exposure
- extremely
- Facility
- familiar
- far
- Features
- few
- field
- finding
- findings
- fit
- food
- For
- Forbes
- Force
- form
- formation
- found
- Foundation
- Foundations
- Frequency
- frequently
- from
- Fuel
- fuels
- functional
- funded
- further
- future
- future of computing
- Gain
- GAS
- generation
- get
- getting
- gif
- Go
- goal
- greenhouse gas
- Group
- had
- Have
- he
- Held
- help
- High
- High Performance Computing
- high-quality
- his
- holds
- HOT
- How
- How To
- However
- http
- HTTPS
- huge
- Hybrid
- hydrogen
- identify
- identifying
- if
- images
- Impacts
- implementing
- important
- improve
- in
- Inc.
- include
- Including
- incredibly
- Indicator
- industrial
- information
- insight
- instead
- integral
- intend
- interact
- interactions
- interacts
- interest
- interesting
- internal
- International
- into
- intriguing
- investigate
- Iowa
- issue
- issues
- IT
- ITS
- jpg
- July
- june
- just
- kidney
- Kills
- Kind
- Know
- knowledge
- laboratory
- larger
- largest
- laser
- lasers
- layers
- lead
- leader
- Leaks
- learned
- Level
- lidar
- light
- like
- Limited
- links
- Look
- LOOKS
- Lot
- made
- Majority
- make
- MAKES
- manage
- management
- many
- Mason
- material
- materials
- Matter
- May..
- means
- measured
- measurement
- measurements
- measures
- mechanical
- mechanics
- mechanisms
- metal
- methane
- method
- Methodology
- methods
- millions
- model
- modeling
- modelling
- molecular
- molecule
- Monitor
- more
- most
- move
- Nanomaterials
- nanotechnology
- National
- Natural
- Natural Gas
- Need
- net
- network
- New
- news
- nexus
- no
- now
- nuclear
- Nuclear Energy
- number
- NY
- obtained
- occur
- of
- off
- Office
- often
- Oil
- on
- online
- only
- opening
- Operations
- or
- order
- Other
- our
- out
- overall
- own
- Paper
- part
- past
- Paving
- performance
- performed
- periodic
- personal
- phase
- PHP
- physical
- Physical Sciences
- plastic
- plato
- Plato Data Intelligence
- PlatoData
- please
- Pollution
- possibilities
- possible
- Post
- posted
- Practical
- precisely
- predict
- Predictions
- preferred
- present
- press
- Press Release
- pressing
- pressure
- pretty
- previous
- principles
- process
- Professor
- Program
- properties
- provided
- publish
- published
- Push
- put
- Quantum
- Quantum Mechanics
- quest
- range
- reaction
- really
- recognized
- recycling
- related
- release
- released
- Releases
- reliable
- rely
- remove
- report
- representation
- research
- researchers
- Resources
- responsible
- resulted
- Results
- return
- reveal
- Revealed
- s
- safe
- safely
- Said
- same
- Save
- saw
- say
- Scale
- Science
- SCIENCES
- scientific
- scientists
- Search
- separate
- seven
- several
- Shape
- Share
- she
- side
- significant
- single
- Snap
- So
- so Far
- solely
- solid
- Solutions
- some
- Soon
- Source
- speaking
- specialized
- Spectroscopy
- spent
- Spot
- Stability
- stabilize
- start
- States
- stored
- strategies
- strength
- strong
- structure
- Student
- Students
- studies
- Study
- submit
- such
- suite
- support
- Supported
- supporter
- Surface
- system
- Systems
- T
- table
- Take
- team
- Technologies
- Technology
- test
- Testing
- than
- that
- The
- The Future
- their
- Them
- then
- theoretical
- theory
- There.
- These
- they
- things
- this
- those
- though?
- thought
- thrilled
- Through
- time
- to
- together
- took
- tool
- top
- topic
- towards
- Training
- transferred
- transport
- Trends
- Trust
- twist
- two
- types
- u.s.
- under
- understand
- understanding
- underwater
- Unexpected
- unique
- United
- United States
- university
- until
- us
- use
- used
- User
- uses
- utilized
- Valuable
- Vast
- Vehicles
- very
- viruses
- Visit
- wanted
- was
- Waste
- Water
- Wave
- Way..
- ways
- we
- WELL
- were
- What
- when
- which
- why
- wide
- Wide range
- will
- with
- within
- Word
- Work
- work out
- working
- world
- would
- Yahoo
- yet
- you
- zephyrnet