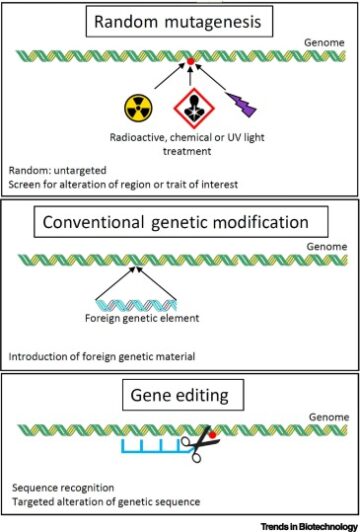
Multi-component hydrogel beads incorporated with reduced graphene oxide for pH-responsive and controlled co-delivery of multiple agents.
Pharmaceutics. 2021; 13: 313
Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs.
Asian J. Pharm. Sci. 2021; 16: 77-85
Non-aromatic clusteroluminogenic polymers: structural design and applications in bioactive agent delivery.
Mater. Today Chem. 2022; 23100712
Noninvasive photoacoustic and fluorescent tracking of optical dye labeled T cellular activities of diseased sites at new depth.
J. Biophotonics. 2018; 11e201800073
Photoacoustic molecular imaging-escorted adipose photodynamic-browning synergy for fighting obesity with virus-like complexes.
Nat. Nanotechnol. 2021; 16: 455-465
Construction and evaluation of red blood cells-based drug delivery system for chemo-photothermal therapy.
Colloids Surf. B Biointerfaces. 2021; 204111789
Red blood cells-derived vesicles for delivery of lipophilic drug camptothecin.
ACS Appl. Mater. Inter. 2019; 11: 22141-22151
Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy.
Sci. Adv. 2019; 5: eaaw6870
Nucleic acid delivery with red-blood-cell-based carriers.
Int. J. Mol. Sci. 2021; 22: 5264
Extracellular vesicles derived from Plasmodium-infected and non-infected red blood cells as targeted drug delivery vehicles.
Int. J. Pharmaceut. 2020; 587119627
Transferrin-conjugated red blood cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for the delivery of doxorubicin and methylene blue.
ACS Appl. Nano Mater. 2020; 3: 3807-3819
Cargo-laden erythrocyte ghosts target liver mediated by macrophages.
Transfus. Apher. Sci. 2021; 60102930
Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application.
Acta Pharm. Sin. B. 2019; 9: 675-689
Haemoglobin-loaded metal organic framework-based nanoparticles camouflaged with a red blood cell membrane as potential oxygen delivery systems.
Biomater. Sci. 2020; 8: 5859-5873
Polyethylene oxide-polyacrylic acid-folic acid (PEO-PAAc) nanogel as a Tc-99m targeting receptor for cancer diagnostic imaging.
J. Labelled Compd. Rad. 2021; 64: 534-547
pH-Responsive biocompatible fluorescent core-shell nanogel for intracellular imaging and control drug release.
Part. Part. Syst. Charact. 2021; 38: 2100110
Ultra-small size gelatin nanogel as a blood brain barrier impermeable contrast agent for magnetic resonance imaging.
Acta Biomater. 2021; 125: 290-299
Design of polymeric gene carriers for effective intracellular delivery.
Trends Biotechnol. 2018; 36: 713-728
A stimuli-responsive nanoparticulate system using poly(ethylenimine)-graft-polysorbate for controlled protein release.
Nanoscale. 2016; 8: 517-528
Mn-dox metal-organic nanoparticles for cancer therapy and magnetic resonance imaging.
Dyes Pigments. 2022; 199110080
Development of copper nanoclusters for in vitro and in vivo theranostic applications.
Adv. Mater. 2020; 32e1906872
Liposome-based delivery of biological drugs.
Chin. Chem. Lett. 2022; 33: 587-596
Combined fluorescence and optoacoustic imaging for monitoring treatments against CT26 tumors with photoactivatable liposomes.
Cancers. 2022; 14: 197
Utilizing sphingomyelinase sensitizing liposomes in imaging intestinal inflammation in dextran sulfate sodium-induced murine colitis.
Biomedicines. 2022; 10: 413
Molecular design of layer-by-layer functionalized liposomes for oral drug delivery.
ACS Appl. Mater. Inter. 2020; 12: 43341-43351
Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting.
J. Control. Release. 2018; 282: 13-24
Vaccine delivery system for tuberculosis based on nano-sized hepatitis B virus core protein particles.
Int. J. Nanomedicine. 2013; 8: 835-843
In vivo nano-biosensing element of red blood cell-mediated delivery.
Biosens. Bioelectron. 2021; 175112845
Red blood cell-derived nanovesicles for safe and efficient macrophage-targeted drug delivery in vivo.
Biomater. Sci. 2019; 7: 187-195
Vascular drug delivery using carrier red blood cells: focus on RBC surface loading and pharmacokinetics.
Pharmaceutics. 2020; 12: 440
Targeted drug delivery to the pulmonary endothelium using liposomal nanocarriers and red blood cell super carriers.
Am. J. Resp. Crit. Care. 2021; 203: 1277752
Erythrocyte ghost based fusogenic glycoprotein vesicular stomatitis virus glycoprotein complexes as an efficient deoxyribonucleic acid delivery system.
J. Biomater. Tiss. Eng. 2022; 12: 1080-1086
A phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study.
Am. J. Hematol. 2015; 90: 811-818
In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase.
Brit. J. Haematol. 2000; 109: 549-554
Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial.
Orphanet. J. Rare Dis. 2014; 9: 5
Erythrocyte encapsulated thymidine phosphorylase for the treatment of patients with mitochondrial neurogastrointestinal encephalomyopathy: study protocol for a multi-centre, multiple dose, open label trial.
J. Clin. Med. 2019; 8: 1096
Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized Phase IIb trial.
Eur. J. Cancer. 2020; 124: 91-101
Erythrocyte membrane concealed paramagnetic polymeric nanoparticle for contrast-enhanced magnetic resonance imaging.
Nanoscale. 2020; 12: 4137-4149
Erythrocyte membrane-camouflaged IR780 and DTX coloading polymeric nanoparticles for imaging-guided cancer photo-chemo combination therapy.
Mol. Pharm. 2019; 16: 3208-3220
An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging.
RSC Adv. 2019; 9: 27911-27926
In vivo positron emission tomographic blood pool imaging in an immunodeficient mouse model using 18F-fluorodeoxyglucose labeled human erythrocytes.
PLoS One. 2019; 14e0211012
Clinical progress and advanced research of red blood cells based drug delivery system.
Biomaterials. 2021; 279121202
Development of long circulating magnetic particle imaging tracers: use of novel magnetic nanoparticles and entrapment into human erythrocytes.
Nanomedicine. 2020; 15: 739-753
Progress on modulating tumor-associated macrophages with biomaterials.
Adv. Mater. 2020; 32e1902007
Heads or tails — what determines the orientation of proteins in the membrane.
FEBS Lett. 1995; 369: 76-79
Surface functionalization of gold nanoparticles with red blood cell membranes.
Adv. Mater. 2013; 25: 3549-3553
Prussian blue nanoparticles operate as a contrast agent for enhanced photoacoustic imaging.
Chem. Commun. 2013; 49: 11029-11031
Fashioning prussian blue nanoparticles by adsorption of luminophores: synthesis, properties, and in vitro imaging.
Inorg. Chem. 2020; 59: 4567-4575
CpG-coated prussian blue nanoparticles-based photothermal therapy combined with anti-CTLA-4 immune checkpoint blockade triggers a robust abscopal effect against neuroblastoma.
Transl. Oncol. 2020; 13100823
Prussian blue nanoparticles: synthesis, surface modification, and biomedical applications.
Drug Discov. Today. 2020; 25: 1431-1443
Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes.
Theranostics. 2017; 7: 523-537
Erythrocyte-derived vesicles for circulating tumor cell capture and specific tumor imaging.
Nanoscale. 2019; 11: 12388-12396
Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy.
ACS Nano. 2017; 11: 3496-3505
Erythrocyte membrane-camouflaged gefitinib/albumin nanoparticles for tumor imaging and targeted therapy against lung cancer.
Int. J. Biol. Macromol. 2021; 193: 228-237
Erythrocyte-derived theranostic nanoplatforms for near infrared fluorescence imaging and photodestruction of tumors.
ACS Appl. Mater. Interfaces. 2018; 10: 27621-27630
Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy.
Biomaterials. 2016; 92: 13-24
Non-invasive photoacoustic imaging of in vivo mice with erythrocyte derived optical nanoparticles to detect CAD/MI.
Sci. Rep. 2020; 10: 5983
Technological value of SPECT/CT fusion imaging for the diagnosis of lower gastrointestinal bleeding.
Genet. Mol. Res. 2015; 14: 14947-14955
In vivo imaging of rat vascularity with FDG-labeled erythrocytes.
Pharmaceuticals. 2022; 15: 292
Near infrared fluorescence imaging of intraperitoneal ovarian tumors in mice using erythrocyte-derived optical nanoparticles and spatially-modulated illumination.
Cancers. 2021; 13: 2544
In vivo imaging of a single erythrocyte with high-resolution photoacoustic microscopy.
Front. Optoelectron. 2015; 8: 122-127
Noninvasive in vivo characterization of erythrocyte motion in human retinal capillaries using high-speed adaptive optics near-confocal imaging.
Biomed. Opt. Express. 2018; 9: 3653-3677
Use of FITC and CFSE labeled erythrocytes for in vivo retinal imaging in non-human primates.
Invest. Ophthalmol. Vis. Sci. 2020; 61: 897
18F-positron-emitting/fluorescent labeled erythrocytes allow imaging of internal hemorrhage in a murine intracranial hemorrhage model.
J. Cerebr. Blood F Met. 2017; 37: 776-786
Erythrocyte-derived nanoparticles as a theranostic agent for near-infrared fluorescence imaging and thrombolysis of blood clots.
Macromol. Biosci. 2018; 18e1700379
Erythrocyte membrane-camouflaged carrier-free nanoassembly of FRET photosensitizer pairs with high therapeutic efficiency and high security for programmed cancer synergistic phototherapy.
Bioact. Mater. 2021; 6: 2291-2302
Aging erythrocyte membranes as biomimetic nanometer carriers of liver-targeting chromium poisoning treatment.
Drug Deliv. 2021; 28: 1455-1465
Flower, R.W. Methods for production and use of substance-loaded erythrocytes for observation and treatment of microvascular hemodynamics, ES2671710T3
Flower, R.W. Methods for production and use of substance-loaded erythrocytes (S-IEs) for observation and treatment of microvascular hemodynamics, US10041042B2
Flower, R.W. Methods for production and use of substance-loaded erythrocytes for observation and treatment of microvascular hemodynamics, EP3372250B1
Flower, R.W. Methods for production and use of substance-loaded erythrocytes for observation and treatment of microvascular hemodynamics, EP2285421B1
Coller, B.S. Method for preparing targeted carrier erythrocytes, US5328840A
Srivastava, S.C. et al. Method and kit for the selective labeling of red blood cells in whole blood with TC-99M, US4755375A
Kato, M. and Hazue, M. Composition for labeling of red blood cells with radioactive technetium, US4313928A
Schneider, D.R. and Bis, K.G. Contras agent having an imaging agent coupled to viable granulocytes for use in magnetic resonance imaging of abscess and a method of preparing and using same, US5045304A
Chao, F. Platelet membrane microparticles, US5332578A
Fischer, T.H. et al. Delivery of micro- and nanoparticles with blood platelets, US8512697B2
Dacorta, J.A. et al. Spray-dried blood products and methods of making same, ES2684130T3
Dacorta, J.A. et al. Spray-dried blood products and methods of making same, CA2757961C
Dacorta, J.A. et al. Spray-dried blood products and methods of making same, AU2010234607B2
Dacorta, J.A. et al. Spray-dried blood products and methods of making same, EP2416790B1
Dacorta, J.A. et al. Spray-dried blood products and methods of making same, US9867782B2
Multi-task joint learning model for segmenting and classifying tongue images using a deep neural network.
IEEE J. Biomed. Health Inform. 2020; 24: 2481-2489
Endoscope image mosaic based on pyramid ORB.
Biomed. Signal. Process. Control. 2022; 71103261
2D/3D multimode medical image registration based on normalized cross-correlation.
Appl. Sci. 2022; 12: 2828
Improved feature point pair purification algorithm based on sift during endoscope image stitching.
Front. Neurorobot. 2022; 16840594
Transcranial focused ultrasound stimulation of periaqueductal gray for analgesia.
IEEE Trans. Biomed. Eng. 2022; ()
Piezoelectric ultrasound energy-harvesting device for deep brain stimulation and analgesia applications.
Sci. Adv. 2022; 8: eabk0159
Tumor origin detection with tissue-specific miRNA and DNA methylation markers.
Bioinformatics. 2018; 34: 398-406
Cell-based drug delivery.
Adv. Drug Deliv. Rev. 2008; 60: 286-295
Factors associated with the performance of carrier erythrocytes obtained by hypotonic dialysis.
Blood Cells Mol. Dis. 2004; 33: 132-140
Preparation and in vitro evaluation of carrier erythrocytes for RES-targeted delivery of interferon-α 2b.
Int. J. Pharm. 2007; 341: 125-133
Dilution technique to determine the hydrodynamic volume fraction of a vesicle suspension.
Langmuir. 2010; 26: 15169-15176
Erythrocyte-mediated delivery of drugs, peptides and modified oligonucleotides.
Gene Ther. 2002; 9: 749-751
pH-responsive polymers for trehalose loading and desiccation protection of human red blood cells.
Biomaterials. 2011; 32: 4443-4449
Robust three-dimensional nanotube-in-micropillar array electrodes to facilitate size independent electroporation in blood cell therapy.
Lab Chip. 2021; 21: 4196-4207
Incorporation of inositol hexaphosphate into intact red blood cells. I. Fusion of effector-containing lipid vesicles with erythrocytes.
Naturwissenschaften. 1979; 66: 563-566
L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL).
J. Control. Release. 2009; 139: 182-189
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(22)00192-5?rss=yes
- 1
- a
- activities
- advanced
- against
- Agent
- agents
- algorithm
- All
- and
- Application
- applications
- Array
- associated
- authors
- barrier
- based
- Biomaterials
- biomedical
- bis
- Bleeding
- blood
- Blue
- Brain
- Cancer
- capture
- care
- carriers
- Cells
- chip
- chromium
- circulating
- combination
- combined
- complex
- contrast
- control
- controlled
- Copper
- Core
- coupled
- deep
- deep neural network
- delivery
- depth
- Derived
- Design
- Detection
- Determine
- determines
- device
- Diagnostic imaging
- Dialysis
- dna
- drug
- Drugs
- during
- effect
- Effective
- efficiency
- efficient
- Elderly
- emission
- encapsulated
- enhanced
- evaluation
- express
- facilitate
- Feature
- fibers
- fighting
- Focus
- focused
- fraction
- from
- fusion
- Ghost
- Gold
- Graphene
- gray
- having
- Health
- High
- high-resolution
- HTTPS
- human
- Hybrid
- image
- images
- Imaging
- in
- Incorporated
- independent
- inflammation
- infusion
- interfaces
- internal
- kit
- Label
- labeling
- learning
- List
- Liver
- loading
- Long
- Making
- medical
- metal
- method
- methods
- mice
- Microscopy
- model
- modified
- MOL
- molecular
- monitoring
- motion
- multiple
- nano
- Nanomedicine
- native
- Near
- negative
- network
- Neural
- neural network
- New
- novel
- Obesity
- obtained
- ONE
- open
- operate
- optics
- organic
- Origin
- Oxygen
- pairs
- part
- particle
- patients
- performance
- phase
- philadelphia
- plato
- Plato Data Intelligence
- PlatoData
- Point
- Polymers
- pool
- potential
- preparing
- process
- Production
- Products
- programmed
- Progress
- properties
- protection
- Protein
- Proteins
- protocol
- Pyramid
- Randomized
- RARE
- RAT
- rbc
- Red
- Reduced
- reduces
- Registration
- release
- research
- resonance
- Results
- robust
- safe
- same
- SCI
- security
- selective
- Sift
- Signal
- single
- Sites
- Size
- specific
- structural
- studies
- Study
- Super
- surf
- Surface
- suspension
- Symptoms
- synergy
- system
- Systems
- Target
- targeted
- targeting
- The
- Therapeutic
- therapy
- three-dimensional
- to
- today
- Tracking
- treatment
- trial
- ultrasound
- use
- value
- Vehicles
- viable
- virus
- volume
- W
- What
- X
- zephyrnet