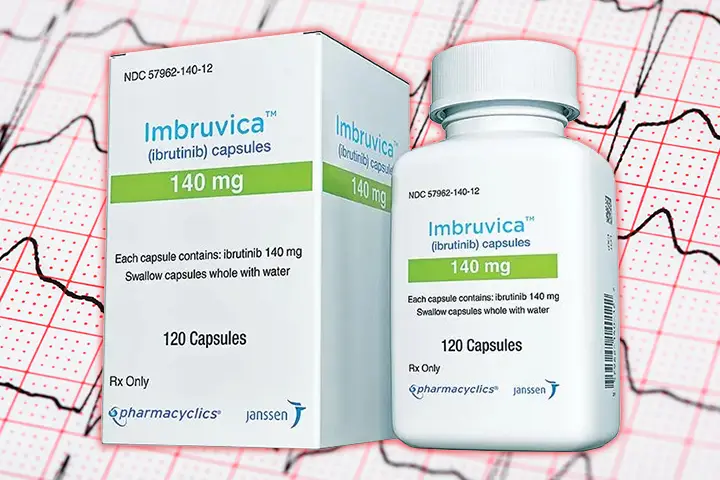
The Ibrutinib Patent Dispute
Adding another chapter to the long-standing legal dispute concerning the Leukaemia medication Imbruvica (API Ibrutinib), the Delhi High Court on December 21, 2023, upheld the IPAB order setting aside the post-grant rejection of the Ibrutinib patent. The Court also restrained Natco Pharma, Hetero, BDR Pharma, Shilpa Medicare, Alkem, and Laurus Labs from manufacturing and marketing the generic versions of Imbruvica. However, considering the importance of the drug, the Court permitted the defendants to exhaust the stock available with them. Pharmacyclics, the plaintiff, is a subsidiary of the US firm AbbVie, while the drug is marketed in India by Johnson & Johnson. The plaintiffs were the licensees of Ibrutinib, which was also being manufactured and sold by several generic medicine companies (defendants), without a license, under various brand names. The Ibrutinib patent is set to expire in 2026.
Readers will recall that this Ibrutinib patent controversy started in 2020 when the Opposition Board rejected patent no. IN262968, covering Ibrutinib, on the basis of Laurus’ post-grant opposition due to a lack of inventive step. In an appeal, the IPAB set aside the impugned rejection and restored the above patent. Meanwhile, prior to the Opposition Board’s order, Pharmacyclics had filed an infringement suit seeking an injunction against the marketing and manufacture of the generic version of Imbruvica. This controversy has previously been discussed on the blog here and here. The current post will focus on 2 issues raised in the current judgement: 1) the validity of the order passed on September 29, 2020 by the Intellectual Property Appellate Board (IPAB) after the retirement of the then Chairperson (retd.) J. Manmohan Singh, and 2) additional arguments seeking revocation of the suit patent. I will then highlight how while making the present finding, the court missed out in properly considering the public interest element involved herein.
Validity of IPAB Orders by Retired Chairperson and Application of the De Facto Doctrine
The validity of orders passed by IPAB after the retirement of the then-chairperson (retd.) J. Manmohan Singh became a significant issue during the final days of the IPAB, as the Chairperson continued to hear matters based on extensions granted by the Supreme Court. This matter has been discussed on the blog multiple times, and you can read about it here, here, and here.
J. Manmohan’s term expired on 21 September 2019, however, the Supreme Court in International Association for Protection of Intellectual Property v. Union of India, dated February 12, 2020, extended this tenure until December 31, 2020. Here, the de facto doctrine becomes of relevance, which provides that the decisions made by an official within the scope of their office, in the public or third-party interest, remain valid and are not considered void even if their own appointment is later found to be invalid or inappropriate.
In light of this, the defendant argued that the incumbent chairperson was incompetent to hold the office due to the expiration of his tenure on September 21, 2019. The defendant relied on Central Bank of India v. Bernard, which provides for the exclusion of the applicability of the de facto doctrine to a usurper in office. The defendant argued that continuing to hold office after retirement would deem the then chairperson as a usurper. Thus, they contended that the judgment pronounced by the Chairman of the IPAB is null and void.
On the other hand, the plaintiff invoked the de facto doctrine, relying on the case Gokaraju Rangaraju v. State of A.P. The DHC agreed to uphold the validity of the IPAB judgment, with J. Hari Shankar stating that it would be “preposterous to hold that the continuance in office of Manmohan Singh, J., which was in accordance with interim orders passed by the Supreme Court in Madras Bar Association, was ‘wrongful’ or that he was holding office as a ‘usurper’.” Compliance with orders passed by the Supreme Court cannot be regarded as wrongful.
A ruling going the other way, could have cast doubt on the validity of all interim IPAB orders, opening a floodgate for appeals challenging IPAB decisions post 20th September 2019.
Authority to Intervene with the IPAB’s Judgement under Writ Jurisdiction
The defendants also challenged the IPAB’s judgement on merits, seeking a stay on its operation. However, the court refused to intervene, citing the limited scope of writ jurisdiction on this issue, and that doing so at an interim stage would amount to restoring status quo ante (something before the existing state of affairs) which has been prohibited by the rationale in Dorab Cawasji Warden v. Coomi Sarab Warden. The DHC noted that, firstly, the judicial review under Article 226/227 is limited to examining jurisdiction errors or decisions fundamentally contrary to law, focusing on the process rather than the merits. Secondly, a stay is granted only if there is: a) a prima facie case, b) balance of convenience, and c) risk of irreparable loss. In light of the above principles, the DHC noted that no grounds for a stay could be established by Laurus’ stay request, and granting one would involve judicially overturning and reverting to the pre-IPAB decision status at an interim stage.
Additional Arguments by the Defendant Seeking Revocation of Suit Patent
After this, the Court moved to the application seeking an interim injunction against the defendants. On this, the defendants raised a defence against the allegation of infringement, questioning the validity of the suit patent.
The defendant argued that- First, the suit patent was covered in a prior US Patent 7459554; Second, the suit patent has been anticipated on the ground of prior publication as it has been disclosed in an article sent for publication by the predecessors (in title) of the plaintiffs on 8 September 2006 (i.e. before the prior date of the suit patent- 22 September 2006), without any confidentiality clause. However, the Court rejected these arguments. For the first argument, the court held that the core moiety in the suit patent and the cited US patent are different, and it cannot determine the extent to which the difference in the core moiety affects the compound’s inhibitory activity, at a prima facie stage. For the second argument, the court held that though the cited article was sent for publication before the priority date of the suit patent, it was eventually published afterward and thus, refused to hold that mere submission of the article will amount to publication.
Affordable Therapy Concerns
Another, and perhaps the most important issue among these is the public interest angle in the judgement. The overwhelming response from patent lawyers, medical professionals, and rights activists has been concerning the blocking across the country, denying patients access to affordable therapy, and the sale of generic versions of this cancer medication. Public interest – which becomes relevant because Ibrutinib is an anti-cancer drug – was not discussed at all in the order passed in 2020 allowing a stay on the drug’s generics.
Justice Hari Shankar observed that the defendants are, in fact, manufacturing and selling Ibrutinib without a licence from the plaintiffs, which is not disputed. Where a granted patent is prima facie found to be infringed and is being exploited without a licence from the patent holder, the balance of convenience is always in favour of restraining further infringement. He acknowledged being aware that the drug in question is needed for treating various serious ailments, including cancer. That said, the court held that the law sternly prohibits patent infringement, and said that it may not be possible to argue that considerations of public interest.
This however goes counter to precedent. There is a fourth-factor test for assessing public interest in pharmaceutical goods. In the case of Roche v. Cipla, the court explicitly declined to issue an interim order based on public interest considerations. Instead, the court directed the defendant to provide an undertaking to pay the damages in case the suit is decreed in favour of the plaintiff. This approach (which has been discussed in depth on this blog before here) could have been applied in the current case as well for a more judicious approach to this issue.
Final Thoughts
Considering the merits of the case, the Court held that there wasn’t any ground to stay the IPAB judgement, holding the suit patent to be valid and restraining the six domestic companies from manufacturing and marketing generic versions of the drug. On the whole, the order is a mixed bag. While the DHC’s upholding of the retired chairman’s order as valid demonstrates judicial propriety in the larger scheme of things, at the same time, it seems to have faltered in sufficiently analysing the application of the fourth-factor test to facilitate public interest ground in granting an injunction on this anti-cancer medication.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://spicyip.com/2024/01/the-ibrunitib-saga-dhc-restrains-generic-competitors-but-what-about-public-interest.html
- :has
- :is
- :not
- :where
- 1
- 12
- 2006
- 2019
- 2020
- 2023
- 2026
- 20th
- 22
- 29
- 31
- 8
- a
- About
- about IT
- above
- access
- accordance
- acknowledged
- across
- activists
- activity
- Additional
- Affairs
- affordable
- After
- against
- agreed
- All
- Allowing
- also
- always
- among
- amount
- an
- analysing
- and
- Another
- Anticipated
- any
- api
- appeal
- appeals
- Application
- applied
- appointment
- approach
- ARE
- argue
- argued
- argument
- arguments
- article
- AS
- aside
- Assessing
- Association
- At
- available
- aware
- b
- bag
- Balance
- Bank
- Bank of India
- bar
- based
- basis
- BE
- became
- because
- becomes
- been
- before
- being
- blocking
- Blog
- board
- brand
- but
- by
- CAN
- Cancer
- cannot
- case
- chairman
- challenged
- challenging
- Chapter
- cited
- citing
- Companies
- competitors
- compliance
- concerning
- confidentiality
- considerations
- considered
- considering
- continued
- continuing
- contrary
- controversy
- convenience
- Core
- could
- Counter
- country
- Court
- covered
- covering
- Current
- Date
- dated
- Days
- December
- decision
- decisions
- decreed
- defence
- defendants
- Delhi
- demonstrates
- depth
- Determine
- difference
- different
- directed
- discussed
- Dispute
- doing
- Domestic
- doubt
- drug
- due
- during
- e
- element
- Errors
- established
- Ether (ETH)
- Even
- eventually
- Examining
- existing
- expiration
- explicitly
- exploited
- extended
- extensions
- extent
- facilitate
- fact
- February
- filed
- final
- finding
- Firm
- First
- Floodgate
- Focus
- focusing
- For
- found
- from
- fundamentally
- further
- Goes
- going
- goods
- granted
- granting
- Ground
- had
- hand
- Have
- he
- hear
- Held
- here
- herein
- High
- Highlight
- his
- hold
- holder
- holding
- How
- However
- HTML
- http
- HTTPS
- i
- if
- importance
- important
- in
- Including
- Incumbent
- india
- infringement
- instead
- intellectual
- intellectual property
- interest
- interim
- intervene
- invoked
- involve
- involved
- issue
- issues
- IT
- ITS
- Johnson
- judicial
- jurisdiction
- Labs
- Lack
- larger
- later
- Law
- Lawyers
- Legal
- Licence
- License
- licensees
- light
- Limited
- long-standing
- loss
- made
- Making
- manufactured
- manufacturing
- Marketing
- Matter
- Matters
- max-width
- May..
- Meanwhile
- medical
- Medicare
- medication
- medicine
- mere
- missed
- mixed
- more
- most
- moved
- multiple
- names
- needed
- no
- noted
- observed
- of
- Office
- official
- on
- ONE
- only
- opening
- operation
- opposition
- or
- order
- orders
- Other
- out
- overwhelming
- own
- passed
- patent
- patent infringement
- patients
- Pay
- perhaps
- Pharma
- Pharmaceutical
- plato
- Plato Data Intelligence
- PlatoData
- possible
- Post
- Precedent
- present
- previously
- principles
- Prior
- priority
- process
- professionals
- prohibited
- pronounced
- properly
- property
- protection
- provide
- provides
- public
- Publication
- published
- question
- raised
- rather
- rationale
- Read
- regarded
- Rejected..
- relevance
- relevant
- relying
- remain
- request
- response
- restored
- restoring
- retirement
- reverting
- review
- rights
- Risk
- ruling
- saga
- Said
- sale
- same
- scheme
- scope
- Second
- seeking
- seems
- Selling
- sent
- September
- serious
- set
- setting
- several
- significant
- SIX
- So
- sold
- something
- Stage
- started
- State
- stating
- Status
- stay
- Step
- stock
- submission
- subsidiary
- Suit
- Supreme
- Supreme Court
- term
- test
- than
- that
- The
- the Law
- their
- Them
- then
- therapy
- There.
- These
- they
- things
- third-party
- this
- though?
- Thus
- time
- times
- Title
- to
- treating
- under
- union
- until
- Uphold
- upholding
- us
- valid
- various
- version
- versions
- was
- Way..
- WELL
- were
- What
- when
- which
- while
- whole
- will
- with
- within
- without
- would
- you
- zephyrnet







![Webinar on “Improving Drug Regulation for Greater Access to Biologics and Vaccines” [December 08]](https://platoaistream.com/wp-content/uploads/2023/12/webinar-on-improving-drug-regulation-for-greater-access-to-biologics-and-vaccines-december-08-211x300.png)