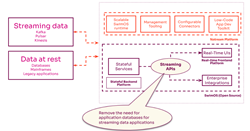
So how can one incorporate different types of data from patients into clinical trials?
TORONTO, Ontario (PRWEB) May 08, 2023
Series Description
The value of real-world data is clear. The acceptance of real-world evidence (RWE) is growing. But is this enough to propel RWE from being a nice-to-have to a must-have? PicnicHealth is hosting a four-part webinar series to reframe the “ROI” for RWE generation and tackle the question: beyond the return on investment for RWE, what is the risk of inaction?
Webinar Description
With increasing applications of real-world data (RWD) upstream in the drug development lifecycle, it’s critical to recognize that there is no one-size-fits-all approach. So how can one incorporate different types of data from patients into clinical trials? Through conversation with a panel of experts from the biopharma industry and health technology companies, the featured speakers will discuss scenarios when patient-mediated, tokenized and/or site-based approaches are warranted. Speakers will share their unique perspectives on the importance of a tailored yet flexible strategy and the potential value this can unlock for researchers and patients alike.
Join today to explore design considerations for incorporating different types of real-world data into clinical trials.
Join Gaelan Ritter, Head of Analytics Innovation and Digital Health, Bristol-Myers Squibb; Andrew Larsen, VP of Partnerships, PicnicHealth; Jim Coutcher, Senior Director, Global Head of Emerging Methods and Solutions, Real World Solutions, IQVIA; and Evelyn Pyper (Moderator), Evidence Strategist, PicnicHealth, for the live webinar on Wednesday, May 24, 2023, at 12pm EDT (5pm BST/UK).
For more information, or to register for this event, visit Real-World Data By Design — Incorporating Different Data Types into Clinical Trials.
ABOUT PICNICHEALTH
PicnicHealth is a healthcare technology company that partners directly with patients to build deep real-world datasets. The company leverages state-of-the-art machine learning, combined with human curation, to port complete medical records into an easy-to-use online application. The platform gives patients unprecedented access to and control over their medical records and, with their consent, the opportunity to contribute this valuable data to further scientific research. Learn more at PicnicHealth.com.
ABOUT XTALKS
Xtalks, powered by Honeycomb Worldwide Inc., is a leading provider of educational webinars to the global life science, food and medical device community. Every year, thousands of industry practitioners (from life science, food and medical device companies, private & academic research institutions, healthcare centers, etc.) turn to Xtalks for access to quality content. Xtalks helps Life Science professionals stay current with industry developments, trends and regulations. Xtalks webinars also provide perspectives on key issues from top industry thought leaders and service providers.
To learn more about Xtalks visit http://xtalks.com
For information about hosting a webinar visit http://xtalks.com/why-host-a-webinar/
Contact:
Vera Kovacevic
Tel: +1 (416) 977-6555 x371
Email: vkovacevic@xtalks.com
Share article on social media or email:
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Minting the Future w Adryenn Ashley. Access Here.
- Buy and Sell Shares in PRE-IPO Companies with PREIPO®. Access Here.
- Source: https://www.prweb.com/releases/real_world_data_by_design_incorporating_different_data_types_into_clinical_trials_upcoming_webinar_hosted_by_xtalks/prweb19323546.htm
- :is
- 2023
- 24
- 250
- 91
- a
- About
- academic
- academic research
- acceptance
- access
- alike
- also
- an
- analytics
- and
- Andrew
- Application
- applications
- approach
- approaches
- ARE
- article
- At
- being
- Beyond
- biopharma
- build
- but
- by
- CAN
- Centers
- clear
- Clinical
- clinical trials
- COM
- combined
- community
- Companies
- company
- complete
- consent
- considerations
- content
- contribute
- control
- Conversation
- critical
- curation
- Current
- data
- datasets
- deep
- Design
- Development
- developments
- device
- different
- digital
- Digital Health
- directly
- Director
- discuss
- drug
- drug development
- easy-to-use
- educational
- emerging
- enough
- etc
- Ether (ETH)
- Event
- Every
- evidence
- experts
- explore
- featured
- flexible
- food
- For
- from
- further
- generation
- gives
- Global
- Growing
- head
- Health
- healthcare
- helps
- hosting
- How
- http
- HTTPS
- human
- image
- importance
- in
- inaction
- Inc.
- incorporate
- incorporating
- increasing
- industry
- information
- Innovation
- institutions
- into
- investment
- issues
- Jim
- Key
- leaders
- leading
- LEARN
- learning
- leverages
- Life
- Life Science
- lifecycle
- live
- machine
- machine learning
- May..
- Media
- medical
- medical device
- methods
- more
- Must-Have
- no
- of
- on
- ONE
- online
- Ontario
- Opportunity
- or
- over
- panel
- partners
- partnerships
- patients
- perspectives
- platform
- plato
- Plato Data Intelligence
- PlatoData
- potential
- powered
- private
- professionals
- Propel
- provide
- provider
- providers
- quality
- question
- real
- real world
- recognize
- records
- register
- regulations
- research
- Research Institutions
- researchers
- return
- Risk
- scenarios
- Science
- Scientific Research
- senior
- Series
- service
- service providers
- Share
- So
- Social
- social media
- Solutions
- speakers
- state-of-the-art
- stay
- Strategist
- Strategy
- tailored
- Technology
- technology companies
- that
- The
- their
- There.
- this
- thought
- thought leaders
- thousands
- Through
- to
- today
- tokenized
- top
- Trends
- trials
- TURN
- types
- unique
- unlock
- unprecedented
- Valuable
- value
- Visit
- webinar
- Webinars
- Wednesday
- What
- What is
- when
- will
- with
- world
- worldwide
- year
- yet
- zephyrnet