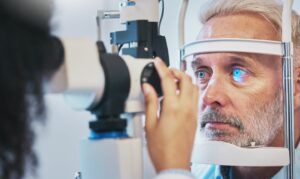
NovaBay Pharmaceuticals has commercially introduced its optic Avenova Allograft for eyecare professionals in the US.
Available through the company’s physician-dispensed channel, the optic allograft can be used as a protective covering during the repair of ocular surfaces.
It is claimed to be the only optic allograft produced utilising the patented six-step BioREtain process from BioStem Technologies.
This process helps protect the natural integrity of the placental tissue. BioStem will handle the manufacturing, packaging, shipping and regulatory compliance of the allograft.
The allograft offers a protective environment or covering to repair the cornea and conjunctiva, enabling the ocular surface to return to a healthier state.
Measuring between 20 and 50μ thick, the allograft features the amnion layer of the placental membrane and is suitable for delicate ophthalmic applications. It is available in 8mm, 10mm and 12mm diameter sizes.
This product launch follows a previous agreement in which NovaBay secured the right to market BioStem’s Amniotic Tissue Allograft under the Avenova brand.
NovaBay CEO and General Counsel Justin Hall said: “Avenova Allograft is a valuable addition to our high-quality, differentiated eyecare franchise and allows us to provide another solution for the professionals who specialise in treating eye conditions.
“We have fostered strong relationships with eyecare professionals nationwide, including thousands of optometrists and ophthalmologists who recommend, prescribe and sell Avenova-branded products every day. Sales in our physician-dispensed channel continue to grow, providing us with a terrific platform to launch this innovative product.”
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.medicaldevice-network.com/news/novabay-pharmaceuticals-introduces-optic-allograft/
- :has
- :is
- 10mm
- 20
- a
- addition
- Agreement
- allows
- and
- Another
- applications
- AS
- available
- BE
- between
- brand
- CAN
- ceo
- Channel
- claimed
- COM
- Company’s
- compliance
- conditions
- continue
- counsel
- covering
- credit
- day
- differentiated
- during
- enabling
- Environment
- Every
- every day
- eye
- Features
- follows
- For
- fostered
- Franchise
- from
- General
- Grow
- Hall
- handle
- Have
- healthier
- helps
- high-quality
- HTTPS
- in
- Including
- innovative
- integrity
- Introduces
- IT
- ITS
- jpg
- Justin
- launch
- layer
- manufacturing
- Market
- Nationwide
- Natural
- of
- Offers
- only
- or
- our
- packaging
- patented
- pharmaceuticals
- platform
- plato
- Plato Data Intelligence
- PlatoData
- prescribe
- previous
- process
- Produced
- Product
- product launch
- Production
- Products
- professionals
- protect
- Protective
- provide
- providing
- recommend
- regulatory
- Regulatory Compliance
- Relationships
- repair
- return
- right
- Said
- sales
- Secured
- sell
- Share
- Shipping
- shutterstock
- sizes
- solution
- State
- strong
- suitable
- Surface
- Technologies
- The
- this
- thousands
- Through
- tissue
- to
- treating
- under
- us
- used
- Valuable
- which
- WHO
- will
- with
- zephyrnet