<!–
–>
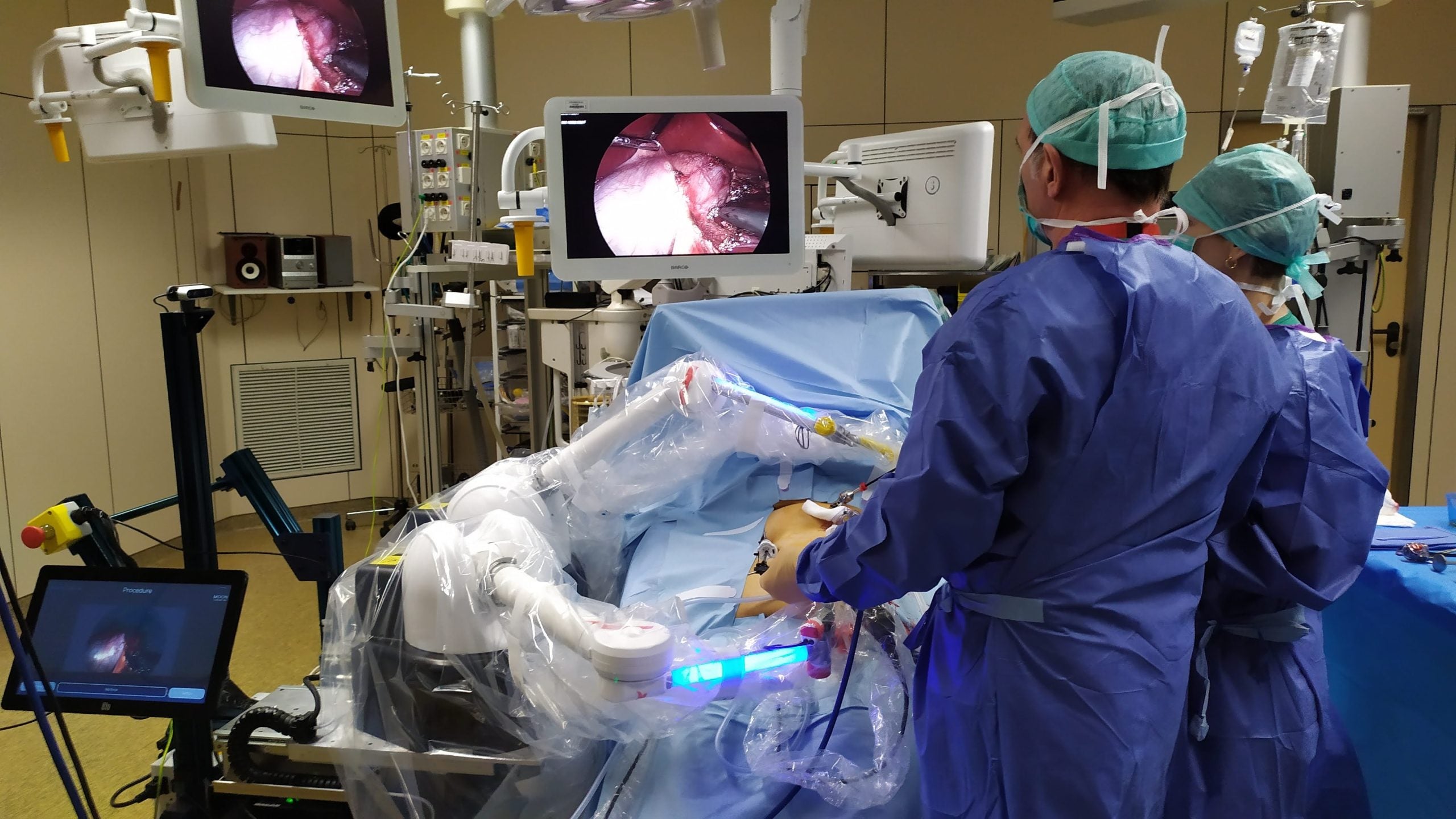
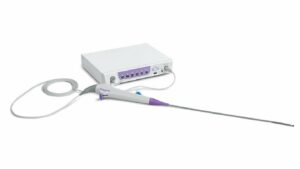
Surgeons in the US have used Moon Surgical’s Maestro system to aid weight loss surgery procedures, in what are the first-use cases of the robotic system in the country.
Maestro, which received US Food and Drug Administration (FDA) clearance in December 2022, is designed to assist in traditional laparoscopic procedures.
Three surgeons at Baptist Health in Florida, US, used the robotic system to help perform sleeve gastrectomies – the most common weight loss surgery. The robotic arms can hold and adjust instruments, reducing the number of staff required in the operating room.
Dr Ron Landmann, chief of colon and rectal surgery and medical director of informatics at Baptist Health said in a statement: “Maestro provides the control and dependability needed in minimally invasive surgery at a time when staffing shortages introduce workflow challenges and variability.”
Moon Surgical recently announced an updated version of the system received CE marking. Moon Surgical said it improved scalability and refined aesthetics, with additional features such as cloud connectivity and surgeon-guided hands-free scope control.
Anne Osdoit, CEO of Moon Surgical said in a statement: “After treating fifty patients in our first-in-human clinical trial, we are excited to be doing clinical cases in the US.”
The robotics industry is growing at a CAGR of 29% and will be worth $568bn by 2030, according to GlobalData. In 2021, GlobalData valued the surgical robotics market at $9.6bn.
Earlier in September, UK robotic surgery group CMR Surgical raised $165m. In May 2023, Moon Surgical raised $55.4m in venture funding.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.medicaldevice-network.com/news/moon-surgicals-maestro-system-used-in-first-us-clinical-procedures/
- :is
- 2021
- 2022
- 2023
- 2030
- a
- Additional
- adjust
- administration
- Aid
- an
- and
- announced
- ARE
- arms
- AS
- assist
- At
- BE
- being
- by
- CAGR
- CAN
- cases
- ceo
- challenges
- chief
- clearance
- Clinical
- Cloud
- Common
- Connectivity
- control
- country
- credit
- December
- designed
- Director
- doing
- drug
- Ether (ETH)
- excited
- fda
- Features
- First
- florida
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- GlobalData
- Group
- Growing
- Have
- Health
- help
- hold
- HTTPS
- image
- improved
- in
- industry
- instruments
- introduce
- invasive
- IT
- jpg
- loss
- Maestro
- Market
- marking
- May..
- medical
- Moon
- most
- needed
- number
- of
- operating
- our
- patients
- perform
- plato
- Plato Data Intelligence
- PlatoData
- procedure
- procedures
- provides
- raised
- received
- recently
- reducing
- refined
- required
- robotics
- RON
- Room
- Said
- Scalability
- scope
- September
- Share
- shortages
- Staff
- staffing
- Statement
- such
- Surgery
- surgical
- system
- The
- this
- time
- to
- traditional
- treating
- trial
- Uk
- updated
- us
- US Food
- used
- valued
- venture
- version
- we
- weight
- What
- when
- which
- will
- with
- workflow
- worth
- zephyrnet