<!–
–>
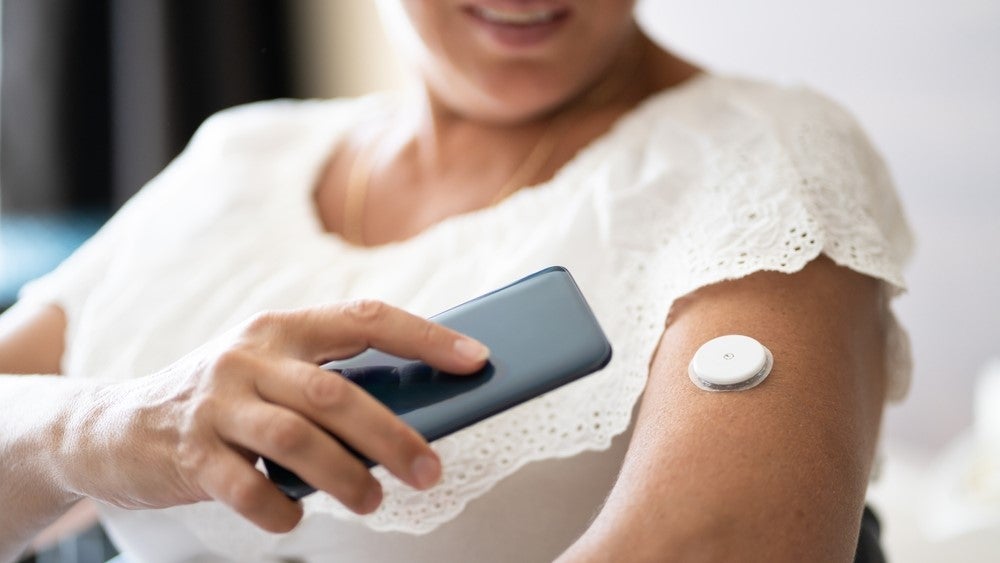
Medtronic has received a CE mark for its continuous glucose monitoring (CGM) device, Simplera, allowing the device to be marketed in the European Union.
The company will begin a phased launch of Simplera at the European Association for the Study of Diabetes (EASD) 59th Annual Meeting in Hamburg (Germany), 2-6 October 2023.
Simplera is a disposable CGM device with a no-fingerstick sensor and can link with Medtronic’s smart insulin pen, InPen.
The InPen system has a reusable smart pen and Bluetooth-enabled app to calculate insulin doses, issue dose reminders, track active insulin and send reports to caregivers. The device was approved by the US Food and Drug Administration (FDA) in 2020.
Diabetes incidences have been steadily increasing over the years. A 2023 study forecasts that over 1.31 billion people will have diabetes by 2050, with most of this population suffering from type 2 diabetes.
The CGM device market is booming and GlobalData forecasts that CGMs will generate $8.4bn in sales by 2030. Market growth drivers include the Covid-19 pandemic, the increasing ease of use of these devices, the need for improved healthcare in rural communities, and the ageing population.
Diabetes is a high-grossing indication for Medtronic, it generated $556m in Q2 2023, as per the company’s financials. However, the company expects its diabetes portfolio to generate more following FDA approvals for insulin and CGM devices.
The FDA lifted the warning letter it placed on Medtronic insulin pumps, including the MiniMed 600 series, after almost two years in April. Medtronic’s insulin pump system with a no-fingerstick sensor, MiniMed 780G was also approved by the FDA in April.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.medicaldevice-network.com/news/medtronics-continuous-glucose-monitor-simplera-receives-ce-mark/
- :has
- :is
- 1
- 2020
- 2023
- 2030
- 2050
- 23
- 31
- a
- active
- administration
- After
- Allowing
- almost
- also
- and
- annual
- app
- approvals
- approved
- April
- AS
- Association
- At
- BE
- been
- begin
- Billion
- by
- CAN
- Communities
- company
- continuous
- COVID-19
- credit
- device
- Devices
- Diabetes
- dose
- drivers
- drug
- ease
- ease of use
- European
- european union
- fda
- following
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- For
- forecasts
- from
- generate
- generated
- Germany
- GlobalData
- Growth
- Have
- healthcare
- However
- HTML
- HTTPS
- image
- improved
- in
- include
- Including
- increasing
- indication
- issue
- IT
- ITS
- jpg
- letter
- Lifted
- LINK
- mark
- Market
- meeting
- Monitor
- monitoring
- most
- Need
- october
- of
- on
- over
- People
- per
- Phased
- plato
- Plato Data Intelligence
- PlatoData
- population
- portfolio
- PRNewswire
- pump
- pumps
- Q2
- received
- receives
- Reports
- reusable
- Rural
- sales
- send
- Series
- Share
- shutterstock
- smart
- Study
- suffering
- system
- that
- The
- These
- this
- to
- track
- two
- type
- Type 2 diabetes
- union
- us
- US Food
- use
- warning
- was
- will
- with
- years
- zephyrnet