<!–
–>
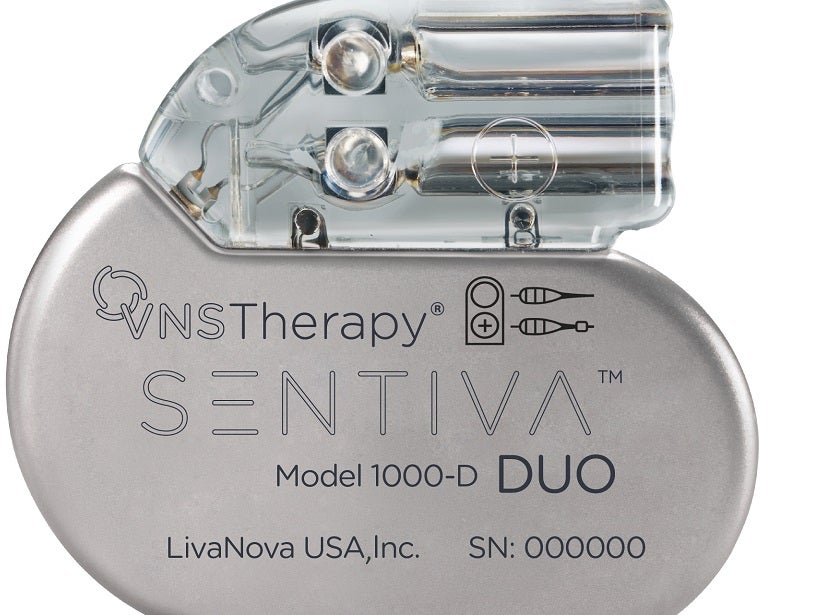
Medical technology company LivaNova has launched SenTiva DUO, a new implantable pulse generator (IPG).
Featuring a dual-pin head, the new IPG is designed to provide Vagus Nerve Stimulation (VNS) therapy for drug-resistant epilepsy treatment.
The SenTiva DUO allows VNS therapy patients who were originally implanted with a dual-pin lead to receive the benefits of the latest technology.
LivaNova said that the SenTiva DUO offers therapeutic benefits that are the same as the original single-pin format SenTiva.
Pediatric neurology chief at Le Bonheur Children’s Hospital, Memphis, Tennessee, US, Dr James Wheless said: “This new offering enables those VNS Therapy patients with legacy dual-pin systems to have the option to replace their device with SenTiva DUO, delivering the most advanced VNS Therapy treatment without the need for lead revision.
“Having access to SenTiva DUO offers patients therapy that can be customised and delivered automatically, providing optimal seizure control and enabling maximum adherence to treatment.”
The two IPGs provide stimulation in response to quick heart rate increases, which may be linked with seizures, offer scheduled as well as day-night programming, and log low heart rate and prone position events.
LivaNova CEO Damien McDonald said: “We’re proud to offer SenTiva DUO to serve patients who were early adopters of VNS Therapy many years ago.
“Over time, all VNS Therapy patients must replace their generators as batteries become depleted. Now, our ‘pioneer patients’ will be able to take advantage of the latest technology with SenTiva DUO and experience the full benefits of VNS Therapy, without the need to replace their dual-pin lead.”
LivaNova’s SenTiva DUO premarket approval (PMA) supplement has received approval from the US Food and Drug Administration (FDA) and is now available in the US.
VNS therapy is designed to treat drug-resistant epilepsy in adults and children as young as four-year-old who have partial-onset seizures.
Last year, LivaNova secured US FDA 510(k) approval for extracorporeal membrane oxygenation (ECMO) for its LifeSPARC system.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.medicaldevice-network.com/news/livanova-sentiva-duo-epilepsy/
- a
- Able
- access
- Ad
- administration
- adopters
- adults
- advanced
- ADvantage
- All
- allows
- and
- approval
- automatically
- available
- batteries
- become
- benefits
- business
- Business Wire
- ceo
- chief
- Children
- company
- control
- credit
- delivered
- delivering
- designed
- device
- drug
- Early
- early adopters
- enables
- enabling
- events
- experience
- fda
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- format
- from
- full
- generator
- generators
- GlobalData
- head
- Heart
- Hospital
- HTTPS
- in
- Increases
- latest
- launched
- launches
- lead
- Legacy
- linked
- Low
- many
- maximum
- MCDONALD
- most
- Need
- New
- offer
- offering
- Offers
- optimal
- Option
- original
- originally
- patients
- plato
- Plato Data Intelligence
- PlatoData
- PLC
- position
- Programming
- proud
- provide
- providing
- pulse
- Quick
- Rate
- receive
- received
- replace
- response
- Said
- same
- scheduled
- Secured
- Seizure
- serve
- Share
- Size
- supplement
- system
- Systems
- Take
- Technology
- The
- their
- Therapeutic
- therapy
- time
- to
- treat
- treatment
- unit
- us
- which
- WHO
- will
- Wire
- without
- year
- years
- young
- zephyrnet