<!–
–>
A gel that stops bleeding in less than 10 seconds can now be used on human wounds, after the US Food and Drug Administration (FDA) cleared the medical device.
Cresilon’s haemostatic gel technology, which is made from plant-based polymers, can stop bleeding without the use of manual pressure and is applied direct from a pre-filled syringe.
The technology has already been in use in veterinary medicine since January 2023. The product, marketed as Vetigel, is in application in veterinary surgical procedures as a single-use, prescription gel.
The New York-based biotech company is calling its human medical device Cresilon Haemostatic Gel (CHG); it will only be available via prescription. CHG is indicated for local management of bleeding wounds such as minor cuts and lacerations.
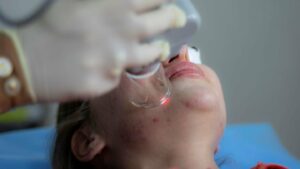
Whilst haemostatic technology itself is not new, its use via a gel is. Most haemostatic agents use a medium of powder or impregnation onto a dressing. A main use of haemostatic gel currently is in dental procedures to stop gingival bleeding. Israeli dental company DSI markets a popular CE-approved gel syringe for this indication.
Cresilon said that although it is the first product approved in its range for human use, the company is targeting future product lines including human surgical applications.
A market growth model by GlobalData estimates that the wound care management market will be worth nearly $39bn by 2030.
“The FDA clearance sets the predicate for our advanced technology and marks Cresilon’s first step towards actualizing our long-term goal of expanding our technology within the broader human health market,” said Cresilon CEO and co-founder Joe Landolina.
According to GlobalData’s deals database, Cresilon has raised $88m in venture financing to date.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.medicaldevice-network.com/news/fda-greenlights-gel-that-stops-bleeding-in-seconds/
- :has
- :is
- :not
- 10
- 2023
- 2030
- 220
- a
- Ad
- administration
- advanced
- Advanced Technology
- After
- agents
- already
- Although
- and
- Application
- applications
- applied
- approved
- AS
- available
- BE
- been
- biotech
- biotech company
- Bleeding
- broader
- by
- calling
- CAN
- care
- ceo
- Co-founder
- company
- credit
- Currently
- cuts
- Date
- Deals
- device
- direct
- drug
- end
- estimates
- expanding
- fda
- financing
- First
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- For
- Forecast
- from
- future
- GlobalData
- goal
- Growth
- Health
- HTTPS
- human
- image
- in
- Including
- indicated
- indication
- Israeli
- IT
- ITS
- itself
- January
- joe
- jpg
- less
- lines
- local
- long-term
- made
- Main
- management
- manual
- Market
- Markets
- medical
- medical device
- medicine
- medium
- minor
- model
- most
- nearly
- New
- New York-Based
- now
- of
- on
- only
- or
- our
- plato
- Plato Data Intelligence
- PlatoData
- Polymers
- Popular
- prescription
- pressure
- procedures
- Product
- raised
- range
- reach
- Said
- seconds
- Sets
- Share
- since
- Size
- Step
- Stop
- Stops
- such
- surgical
- targeting
- Technology
- than
- that
- The
- this
- to
- towards
- unit
- us
- use
- used
- venture
- veterinary
- via
- which
- will
- within
- without
- worth
- wounds
- zephyrnet