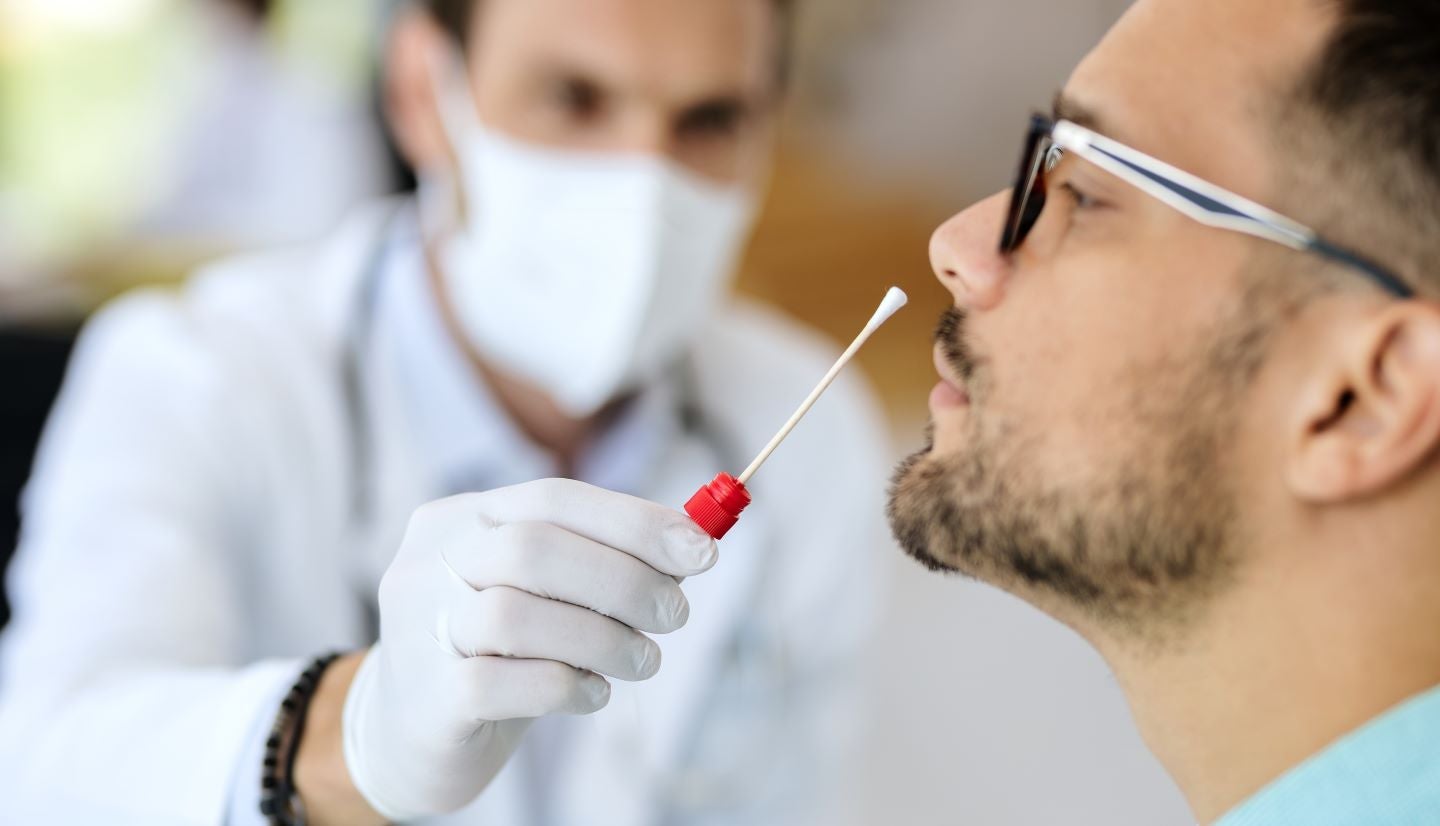
Molecular diagnostics company Co-Diagnostics (Co-Dx) has sought emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for its Co-Dx PCR Covid-19 test with Co-Dx PCR Pro instrument.
Co-Dx’s submission for review by the FDA includes the PCR Pro instrument, Covid-19 detection test and a mobile application, all custom-made for point-of-care and at-home use.
The test kit operates on the real-time polymerase chain reaction (PCR) Co-Primers technology and has demonstrated the capability to detect Covid-19 from anterior nasal swab samples in clinical evaluations.
Furthermore, it showed to deliver results on the smartphone or mobile device of the user in nearly 30 minutes.
The company’s portfolio of future tests presently in development for the new platform comprises tuberculosis (TB), human papillomavirus (HPV), and a multiplex respiratory panel capable of detecting influenza A/B, Covid-19, and respiratory syncytial virus (RSV) from a single sample.
These three tests have received grant from various funding organisations over the past six months this year.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataVarious regulatory agencies including the FDA are presently reviewing the comprehensive Co-Dx PCR platform comprising PCR Home, PCR Pro, mobile app and the test kit is not yet commercially available.
Co-Diagnostics CEO Dwight Egan said: “This new platform technology is a significant step towards advancing the company’s mission to increase accessibility of PCR diagnostics.
“In addition to the development of new technologies from the ground-up by a world-class team to decentralise PCR diagnostics technology and make it available at the point-of-care and in at-home settings, it also required the new technology to be able to be commercialised at a price point that is relevant worldwide.
“Diagnostics, along with vaccines and therapeutics, are a vital tool in helping to combat illnesses like TB, which remains a significant problem in India and many other countries despite being a highly treatable disease.”
The company focuses on developing and commercialising diagnostics technologies.
These technologies are leveraged by tests that detect and/or assess the nucleic acid molecules, including DNA and RNA.
In November this year, the company received a grant worth $8.97m from the Bill & Melinda Gates Foundation to support the development of a TB test.
<!-- -->- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.medicaldevice-network.com/news/co-dx-fda-eua-covid-test/
- :has
- :is
- :not
- $UP
- 11
- 30
- 7
- 8
- 9
- a
- Able
- About
- accessibility
- addition
- administration
- advancing
- agencies
- All
- along
- also
- an
- and
- app
- Application
- ARE
- article
- assess
- At
- authorization
- available
- banner
- BE
- being
- below
- beneficial
- Bill
- business
- by
- CAN
- capability
- capable
- ceo
- chain
- Clinical
- COM
- combat
- commercially
- company
- Company’s
- competitive
- comprehensive
- comprises
- comprising
- confident
- countries
- COVID-19
- credit
- daily
- Daily news
- decision
- deliver
- deliver results
- demonstrated
- Despite
- detect
- Detection
- developing
- Development
- device
- diagnostics
- Disease
- dna
- download
- drug
- Edge
- emergency
- end
- Ether (ETH)
- evaluations
- fda
- focuses
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- For
- form
- Foundation
- Free
- from
- funding
- future
- Gain
- Gates
- GlobalData
- grant
- Have
- helping
- highly
- Home
- HOURS
- However
- HTTPS
- human
- ICON
- in
- includes
- Including
- Increase
- industry
- Industry Insights
- Influenza
- insights
- instrument
- IT
- ITS
- jpg
- kit
- leading
- leveraged
- like
- make
- many
- Market
- melinda gates
- minutes
- Mission
- Mobile
- Mobile app
- mobile device
- months
- most
- nasal
- nearly
- New
- New Platform
- New technologies
- news
- Newsletter
- November
- of
- offer
- on
- operates
- or
- Organisations
- Other
- our
- Our Company
- over
- panel
- past
- PCR
- platform
- platform technology
- plato
- Plato Data Intelligence
- PlatoData
- Point
- portfolio
- powered
- presently
- price
- Pro
- Problem
- Profile
- Profiles
- quality
- reaction
- real-time
- received
- regulatory
- relevant
- remains
- required
- research
- Results
- review
- reviewing
- RNA
- RSV
- Said
- Save
- Seeks
- settings
- showed
- shutterstock
- significant
- single
- SIX
- Six months
- smartphone
- So
- sought
- start
- Step
- submission
- support
- SVG
- team
- Technologies
- Technology
- test
- tests
- that
- The
- therapeutics
- this
- this year
- three
- to
- tool
- towards
- unique
- us
- US Food
- use
- User
- vaccines
- various
- virus
- vital
- want
- we
- which
- will
- with
- world-class
- worldwide
- worth
- year
- yet
- you
- Your
- zephyrnet