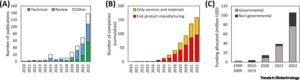
Highlights
-
High-definition (HD) bioprinting enables spatial resolution on a cellular and subcellular level in 3D, allowing reproduction of key features of the cellular microenvironment at a scale not achievable with conventional bioprinting techniques, and allowing control of material properties, geometry, and chemical and physical properties of cell-containing constructs.
-
Light-based, precision jetting, and electrohydrodynamic technologies can already achieve such resolution, and will be increasingly applied to engineer disease models, organ-on-a-chip devices, and implantable microscaffolds with high complexity.
-
Standing challenges include preserving microscale and submicroscale resolution while enabling high-throughput and volumetric construct bioprinting, streamlined multimaterial processing, and the development of new functional (bio)inks and (bio)resins.
Abstract
Bioprinting aims to produce 3D structures from which embedded cells can receive mechanical and chemical stimuli that influence their behavior, direct their organization and migration, and promote differentiation, in a similar way to what happens within the native extracellular matrix. However, limited spatial resolution has been a bottleneck for conventional 3D bioprinting approaches. Reproducing fine features at the cellular scale, while maintaining a reasonable printing volume, is necessary to enable the biofabrication of more complex and functional tissue and organ models. In this opinion article we recount the emergence of, and discuss the most promising, high-definition (HD) bioprinting techniques to achieve this goal, discussing which obstacles remain to be overcome, and which applications are envisioned in the tissue engineering field.
Keywords
Engineering the 3D cellular microenvironment
], cell migration [
], and tissue and organ development [
]. In particular, the ECM has been extensively demonstrated to steer cell and tissue function during tissue morphogenesis and development, and tissue regeneration, as well as in degenerative diseases and cancer, as cells sense local variations in mechanical properties, molecular composition, and 3D (micro)architecture [
] (Figure 1). For example, it was recently demonstrated that the composition of the hydrogel matrix – that mimics the mechanical properties and chemical composition of the ECM– plays a decisive role in replicating morphogenic events allowing intestinal organoid maturation in vitro [
]. Furthermore, creating ~30 μm wide regions of softer hydrogel next to an embedded intestinal stem-cell colony enabled precise control of its shape and the formation of characteristic crypt-like buds [
]. Such spatiotemporal control of intestinal organoid shape on a microscale gave rise to a characteristic distribution of cells (Figure 1B,C). Likewise, inspired by the notion that cells align and accommodate their shape in agreement with the orientation (or lack of thereof) of ECM fibrous structures in vivo [
], topographical elements and matrix mechanical properties at the micro and submicron scales have been extensively studied in the past two decades [
], resulting in the production of biomaterials and cell-laden constructs capable of giving directionality to tissue regeneration, mimicking the anisotropic architecture of tissues (i.e., in skeletal and cardiac muscle) [
], and boosting stem-cell differentiation [
]. Phenotypic transition of fibroblasts to myoblasts is another important phenomenon associated with mechanobiological mechanisms. A recent study demonstrated a predominant role for the directionality of the mechanical signals from the ECM rather than their intensity [
]. Cells interacted with the surrounding collagen fibers to develop tension anisotropy in a two-way cell–ECM feedback. Only cell pretreatment with protrusion-inhibiting nocodazole led to significantly reduced collagen alignment (Figure 1F,G). Most often, these phenomena are investigated on 2.5D patterned surfaces, that is, surfaces with low aspect ratio features, or whose features are in contact with only one side of the cell, produced using conventional replica molding and lithographic techniques [
]. While these methods are highly versatile, allow high resolution (down to hundreds of nanometers) and are compatible with high-throughput screening of broad arrays of topographical features [
], they do not provide sufficient freedom for realizing arbitrary 3D structures typical of native cell-laden environments.

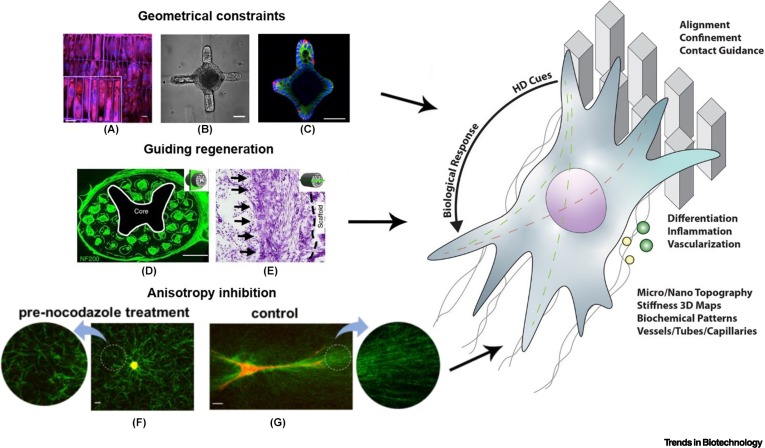
Figure 1Schematic overview of the potential of high-definition (HD) bioprinting for controlling cell fate.
]); (B,C) intestinal organoid with crypt-like buds and characteristic distribution of cells resulting from hydrogel patterning (scale bar: 30 μm) (adapted with permission from [
]); (D,E) neural-progenitor-cell-loaded scaffold representing a complex spinal structure fabricated using DLP-like technology (scale bars: 500 μm and 200 μm) (adapted with permission from [
]); (F,G) effect of pretreatment with nocodazole to inhibit cell protrusions that results in anisotropic collagen fiber alignment (F) compared to an untreated control ((G) adapted with permission from [
]). The schematic on the right side of the figure represents typical extracellular matrix (ECM) mimetic elements that can be reproduced via HD bioprinting (such as micro- and nano-pillars and fibers, among others), and that have in turn been demonstrated to directly influence cell behavior, such as migration, differentiation, secretion of proregenerative or inflammatory molecules, alignment, and the formation of ordered cell assemblies (i.e., as in capillary vessels, and neuronal networks, among others).
3D bioprinting technologies, on the other hand, have increasingly gained relevance in the fields of biomedical and tissue engineering, due to their ability to reproduce complex architectures, potentially mimicking the arrangement of cells as well as structural features of biological tissues and organs. As such, they hold the unique potential to shape the microenvironment into which the cells are brought and to direct their fate. In this opinion article we underline the great potential of 3D bioprinting as a tool to face this challenge, especially through its affirmation and further development of HD-capable technologies. To do so, we provide here an overview of the recent progress in the field, discuss the most promising applications that will likely be enabled by HD bioprinting, and highlight some relevant critical aspects that still need attention from the research and development community.
HD bioprinting techniques to reach single-cell resolution
]. On the downside, printing fidelity for centimeter-sized structures is still not better than 500 μm.
HD bioprinting, in the context of tissue engineering and biofabrication, can be defined as the capability to consistently produce 3D structures with feature sizes below 50 μm, using materials containing cells. In this definition we include also techniques where the material is not deposited line by line or layer by layer, but also those where 3D scanning is performed within a predeposited volume. This includes crosslinking of part of the volume, but also other photochemical effects that result in a density change in the matrix by creating or cleaving bonds in the backbone of the material. Among the available HD bioprinting techniques, multiphoton lithography (MPL) displays the finest resolution to date (<1 μm) that allows crosslinking or other structural modifications within transparent cell-containing materials. Features smaller than the mammalian cell have recently been reproduced via cell electrowriting (EW), which can deposit thin filaments (5 μm) of cell-containing hydrogels. Other bioprinting techniques show a good potential to enter the HD domain, including vat polymerization-based approaches such as digital light processing (DLP), stereolithography (SLA), and volumetric (bio)printing (VP). These techniques are rapidly improving their resolutions, respectively by decreasing the printed layer thickness, pixel size, or single line size, and by the development of advanced tomographic reconstruction algorithms for improved contrast and more accurate light dosage distribution. Finally, methods that allow manipulation and dispensing of minute volumes (a few pl) of cell-laden materials, like laser-induced forward transfer and inkjet bioprinting, are finding new strategies to print well-formed and mechanically stable high-aspect ratio structures, to replicate their high resolution also in the vertical direction.
,
]. This is in part because the lateral resolution is often limited by the photochemistry of the crosslinking process rather than by the minimum laser spot or pixel size, while layer thickness is inherently bound to the light penetration depth. For instance, researchers working with a micrometer-resolution DLP found that, despite the theoretical optical capabilities of their setup, good-quality features were obtained only from a lateral dimension of 100 μm and vertical dimension of 300 μm [
], while many authors reported a layer spacing of between 40 and 50 μm on hydrogels [
,
,
]. In a recent work, Bhusal and colleagues showed lines of 15 μm thickness (Figure 2A ) using cell-free polyethylene glycol diacrylate (PEGDA) hydrogel [
], which is already close to cell size. In general, DLP resolution is also a trade-off between the desired feature size and the sample size, both bound to projector resolution and optics used. An interesting variation, based on the combination of lightsheet excitation and DLP with a dual-color photoinitiator system, could reduce the layer thickness and eliminate the need for layer deposition, reaching 25 μm horizontal and 50 μm vertical resolution in a cell-free resin [
].

Figure 2Feature size of high-definition (HD) bioprinting techniques.
]). (B) Comparison between extrusion-based bioprinted and electrowritten cell-laden gelatin-based hydrogel single fibers (scale bar: 20 μm) (adapted with permission from [
]). (C) Locally two-photon densified lines in a partially ultraviolet-crosslinked gelatin-based hydrogel shows cell alignment along the patterned features (bottom panel) and round cell shape far from them (top panel) (scale bars: 20 μm and 50 μm) (adapted with permission from [
]). (D) Two-photon polymerized gelatin-based hydrogel with hollow channels of 30, 20, and 10 μm diameter. Left panel: 3D reconstruction of the channel network; the yellow signal originates from a fluorescent dye perfusing the channels. Right panel: channels printed in adipose-stem-cell-laden hydrogel to promote the invasion of endothelial cells from a spheroid (red fluorescent) (scale bar: 100 μm) (adapted with permission from [
]). (E) Adipose stem cells migrated from a spheroid into loop channels cleaved around it, at different laser power (30–100 mW) (scale bar: 100 μm) (adapted with permission from [
]). (F) Patterned neutravidin (dotted squares areas) promotes cell adhesion and enables bonding of bone morphogenetic protein-2 (BMP-2) that promotes Smad signaling (visible by bright spots in the cell nuclei in the first two panels of the first row), compared to the control with cell medium (scale bar: 20 μm) (adapted with permission from [
]). Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
,
]. This is of particular interest for biofabrication, where the absence of any shear stresses is beneficial for cell viability. To date, VP has demonstrated feature sizes down to ≈40 μm, and realization of cell-laden and even complex organoid-laden biomaterials with >90% viability [
]. Current challenges regarding the maximum attainable resolution are mainly due to intrinsic losses of reconstruction information, resulting from the back-projection algorithms employed, diffusion of reactive species in the material during crosslinking, and material-dependent fragility of the as-printed part. Minimizing optical attenuation, aberrations, and scattering becomes crucial for accurate results. In cell-laden resins, where cells cause scattering, techniques such as refractive index matching [
] and software-level corrections of the tomographic projections [
] can be effectively employed. Such mitigation strategies are still an active area of research.
,
] and laser-induced forward transfer (LIFT) [
,
] are two patterning techniques that exploit the deposition of small cell-containing material droplets. Despite the nominally high lateral resolution, they generally struggle to produce thick and high-aspect-ratio structures with the same precision, because the bioinks used are usually unable to (i) crosslink quickly after deposition, and (ii) support multiple layers. A few strategies have been proposed to overcome this issue, such as combining the inks with crosslinkers [
], or print at the surface of a crosslinker bath [
].
], even below 100 nm of lateral resolution with special near-field setups [
]. EW has been applied also to water-rich hydrogels [
,
], and recently it was demonstrated to print subcellular size fibers (≈5 μm) with cell-laden hydrogel-based bioinks (cell EW, Figure 2B) [
].
,
,
,
], building simple organ models [
,
,
] (Figure 2D,F), and even in situ printing within living animals (rodents) [
]. Notably, besides conventional polymerization, MPL enables photodegradation and cleaving of sacrificial moieties [
], molecule grafting [
], and local densification of a partially crosslinked hydrogel matrix [
] (Figure 2C,E).

Figure 3Bioprinting techniques: throughput versus resolution.
Throughput versus resolution plot for the bioprinting techniques reviewed in this opinion article: digital light processing (DLP) and stereolithography (DLP/SLA), extrusion bioprinting (EBP), electrowriting (EW), multiphoton lithography (MPL), and volumetric printing (VP). MPL and EW can produce features the size of cells and smaller, controlling the microenvironment at the cell level, but are generally limited to a few mm3/h at most. DLP/SLA, VP, and EBP can easily produce structures of several cm3, with minimal feature size comparable to the diameters of capillaries, cell spheroids, or even blood vessels. Minimum feature size has been extracted from papers cited in the manuscript indicating the experimental line or feature size. Printing throughput, when not directly reported, has been calculated either as volume over printing time, or as line cross-section or lines spacing times linear printing velocity. This could lead for some works to throughput estimations that are slightly off, but nevertheless they should yield the correct order of magnitude, and are the only way to allow a comparison. It was not possible to obtain the necessary data from the papers on inkjet printing. Studies involving cell encapsulation during the printing process are denoted by a cell-shaped datapoint.
Research and clinical applications enabled by HD bioprinting
,
]. Compared to photolithography and replica-based techniques, which typically generate 2.5 D geometries, HD bioprinting techniques produce fully 3D structures, providing a better mimicry of tissue architectures. Notably, light-based technologies can produce structures even across transparent tissue culture labware. Bioprinting directly within a microfluidic chip becomes possible [
,
,
,
], hence allowing the constructs to be fabricated noninvasively, avoiding sterility issues, and reducing the number of assembly steps. Faster development cycles can be enabled by HD bioprinting in the domain of organ-on-a-chip, by producing in a standard chip different internal 3D structures at each redesign and optimization step. Also, the biofabricated constructs can be manipulated in a contactless fashion at multiple time points, adding time as a relevant parameter to mimic the evolution of biological processes [
]. At present, as application fields or markets for HD bioprinting, we can envision the production of organ-on-a-chip for basic/fundamental research, personalized drug testing and diagnostics, and microstructured tissue engineering scaffolds for implantation.
,
,
]. HD bioprinting allows recreation of a highly organized heterogeneous microenvironment with multiple cell types, where the fine features are produced directly instead of relying on microfabricated molds; examples include the study of microvasculature using direct embedding of cells via MPL [
], the creation of semipermeable barriers to mimic the placenta [
], and the use of micro-SLA for an HD approach towards cell patterning to study soluble cell niche factors [
]. Inkjet printing has also been demonstrated for the bioprinting of a heart-on-a-chip [
] to test hydrogel formulations in the context of cardiac tissue response to different drugs. In the future, organ-on-a-chip devices could lead to point-of-care diagnostics and even personalized in vitro drug testing, on clinically relevant models built with patient-specific cells.

Figure 4High-definition (HD) bioprinting targets.
Schematic illustration of the range of locations that HD bioprinting can target. These include elements with fine features and multiple tissues, both inside the human body for implants, and as organs-on-a-chip.
,
]. A few examples of microstructured tissue scaffolds for implantation have been demonstrated through MPL, even though they could not be properly labeled as bioprinting, since cells were seeded or invaded them after printing [
,
,
]. Intravital bioprinting using MPL has also been demonstrated, in which a cell-laden hydrogel precursor is injected subcutaneously, and subsequently patterned and cross-linked to create a scaffold for cell growth directly inside a living animal, leveraging the deep tissue penetration typical of the infrared light used in MPL [
]. At larger scales, HD bioprinting techniques such as microscale continuous projection (based on DLP) have been demonstrated to produce cell-laden scaffolds for central nervous system repair, with promising in vivo results in rescuing spinal cord alignment and connectivity in a murine model [
].
Future directions: higher throughput and tailored bioinks
Alongside the benefits of resolving minute, cell-instructive microscale and nanoscale features, upcoming challenges for future research include preserving such fine resolution while contextually enabling the fabrication of clinically relevant size (centimeter-scale) objects. This is especially relevant, as high resolution is often associated with long fabrication timescales, and cell viability could thus be impaired over lengthy fabrication processes. While in projection and scanning-based techniques the trade-off between resolution and fabrication speed depends on the optics used, in extrusion and droplet printing the fabrication speed is determined by the translation system itself, usually much slower than an optical scanner due to its inertia.
MPL is the HD bioprinting technique that offers the highest resolution, but is significantly slower than other optics-based bioprinting techniques such as DLP, SLA, and VP. This is due to the fact that printing smaller lines or voxels (i.e., volumetric pixels, the smallest polymerized volume) implies the need for a much higher number of them to fill the same volume. Researchers found different strategies to mitigate this issue, increasing the throughput without compromising on the resolution.
]. The use of resonant scanners can further boost the scanning speed [
]; high scanning speed also requires the development of efficient water-soluble photoinitiators with low cytotoxicity to be suitable for bioprinting [
].
]. A similar approach has recently been demonstrated to substantially boost the throughput when processing specialized hydrogels in the presence of living cells [].
], while a spatial light modulator can be used when the foci pattern has to be varied dynamically during the fabrication [
].
The emergence of techniques that address the whole printing volume at once, such as volumetric (bio)printing via tomographic and holographic approaches, can address this problem, as they already allow extremely rapid fabrication rates, while needing to tackle the challenge of approaching the resolution of (sub)micron-level features. Similar observations are valid also for conventional layer-by-layer DLP printing. Primarily, this would require advances in the projection technology; the currently widespread 1080p chips will be replaced by commercial 4K (4x) and 8K (16x) chips, and it can be envisioned that custom-made laboratory prototypes with higher resolution could be produced. That will enable achieving a lateral feature size closer to the diffraction limit, without sacrificing the part volume and printing time.
,
,
], as well as multivat DLP stations, have recently been proposed [
]. As these may still face limitations in terms of throughput and potential loss of valuable cells and resins, further development towards new approaches to enable rapid multimaterial processing in vat polymerization and HD bioprinting will be crucial in the future.
,
,
,
]. Processability and biocompatibility are necessary conditions for bioprinting, but they are not the only properties that could be controlled in a biomaterial; specific molecules and motifs should be added to the materials to elicit a response from the encapsulated cells. An interesting approach is the use of recombinant materials that replicate the structure of natural proteins such as elastin and collagen with high reproducibility, purity (and therefore improved safety), and strongly reducing batch-to-batch variations. Being bioengineered proteins, these can be custom-modified to include other functional components, for example arginine–glycine–aspartate (RGD) peptides to increase cell adhesion [
,
,
], and photoactive moieties to be processed through MPL [
]. Another strategy to improve biofunctionalization consists in the use of protein-adhesive resins (by grafting binding domains, that is, via MPL or molecular imprinting methods) that promote coating from specific biological molecules onto biofabricated scaffolds [
,
].
Concluding remarks and future perspectives
,
] and on artificial barriers [
,
] will enable the study of larger size multitissue organoids.
Regenerative medicine can benefit from the HD bioprinting capability of building cell-laden scaffolds with cell-scale precision, to create implantable artificial tissues with cell guiding, stimulation, and differentiation induction properties. To do so, centimeter-scale printing volume while maintaining fine features must be achieved, either by upscaling HD bioprinting techniques, or improving the resolution of existing high-throughput ones.
How much of a tight control is needed on the microcellular environment to drive successful tissue mimicry and regeneration? What is the lowest resolution limit we should aim for? Which new classes of materials could further improve the realization of more sophisticated organs-on-a-chip and engineered grafts for regenerative medicine?
Acknowledgments
R.L. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 949806, VOLUME-BIO) and from the European’s Union’s Horizon 2020 research and innovation program under grant agreement No 964497 (ENLIGHT). A.O. acknowledges funding from the European Research Council (ERC) (Grant agreement numbers 307701, LeBMEC and 772464, THIRST). The authors would like to thank Professor V. Mironov for fruitful discussions.
Declaration of interests
A.O. is also a Co-Founder and CSO of UpNano GmbH, a recent spin-off of the TU Wien aiming at commercialization of MPL.
References
-
Laminins in epithelial cell polarization: old questions in search of new answers.
Cold Spring Harb. Perspect. Biol. 2017; 9a027920
-
Topotaxis: a new mechanism of directed cell migration in topographic ECM gradients.
Biophys. J. 2018; 114: 1257-1263
-
Probing cellular response to topography in three dimensions.
Biomaterials. 2019; 197: 101-118
-
Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture.
Nat. Protoc. 2017; 12: 2263-2274
-
Tissue geometry drives deterministic organoid patterning.
Science. 2022; 375: eaaw9021
-
A blueprint of the topology and mechanics of the human ovary for next-generation bioengineering and diagnosis.
Nat. Commun. 2021; 12: 5603
-
Biomaterials with structural hierarchy and controlled 3D nanotopography guide endogenous bone regeneration.
Sci. Adv. 2021; 7: eabg3089
-
Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel.
Biofabrication. 2018; 10025003
-
“Raspberry” hierarchical topographic features regulate human mesenchymal stem cell adhesion and differentiation via enhanced mechanosensing.
ACS Appl. Mater. Interfaces. 2021; 13: 54840-54849
-
Tension anisotropy drives phenotypic transitions of cells via two-way cell-ECM feedback.
bioRxiv. 2022; ()
-
Expanding biomaterial surface topographical design space through natural surface reproduction.
Adv. Mater. 2021; 33: 2102084
-
Multiphoton microfabrication and micropatternining (MMM)-based screening of multiplex cell niche factors for phenotype maintenance – bovine nucleus pulposus cell as an example.
Biomaterials. 2022; 281121367
-
3D bioprinting of collagen to rebuild components of the human heart.
Science. 2019; 365: 482-487
-
DLP printing photocurable chitosan to build bio-constructs for tissue engineering.
Carbohydr. Polym. 2020; 235115970
-
From shape to function: the next step in bioprinting.
Adv. Mater. 2020; 32: 1906423
-
Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing.
Nat. Commun. 2018; 9: 1620
-
4D-bioprinted silk hydrogels for tissue engineering.
Biomaterials. 2020; 260120281
-
High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins.
Mater. Today Bio. 2021; 12100162
-
Multi-material digital light processing bioprinting of hydrogel-based microfluidic chips.
Biofabrication. 2021; 14014103
-
Xolography for linear volumetric 3D printing.
Nature. 2020; 588: 620-624
-
High-resolution tomographic volumetric additive manufacturing.
Nat. Commun. 2020; 11: 852
-
Volumetric bioprinting of complex living-tissue constructs within seconds.
Adv. Mater. 2019; 31: 1904209
-
Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories.
Adv. Mater. 2022; 34: 2110054
-
Light-based volumetric additive manufacturing in scattering resins.
arXiv:2105.14952 [physics]. 2021; ()
-
A bioprinted heart-on-a-chip with human pluripotent stem cell-derived cardiomyocytes for drug evaluation.
Bioengineering. 2022; 9: 32
-
Engineering inkjet bioprinting processes toward translational therapies.
Biotechnol. Bioeng. 2020; 117: 272-284
-
Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting.
J. Mater. Sci. Mater. Med. 2019; 30: 28
-
Capillary-like formations of endothelial cells in defined patterns generated by laser bioprinting.
Micromachines. 2021; 12: 1538
-
Enabling free-standing 3D hydrogel microstructures with microreactive inkjet printing.
ACS Appl. Mater. Interfaces. 2020; 12: 1832-1839
-
Femtosecond laser induced densification within cell-laden hydrogels results in cellular alignment.
Biofabrication. 2019; 11035005
-
Melt electrospinning today: an opportune time for an emerging polymer process.
Prog. Polym. Sci. 2016; 56: 116-166
-
Near-field electrospinning for three-dimensional stacked nanoarchitectures with high aspect ratios.
Nano Lett. 2020; 20: 441-448
-
Melt electrospinning writing of poly-hydroxymethylglycolide-co-ε-caprolactone-based scaffolds for cardiac tissue engineering.
Adv. Healthc. Mater. 2017; 6: 1700311
-
A versatile biomaterial ink platform for the melt electrowriting of chemically-crosslinked hydrogels.
Mater. Horiz. 2020; 7: 928-933
-
Hydrogel-based bioinks for cell electrowriting of well-organized living structures with micrometer-scale resolution.
Biomacromolecules. 2021; 22: 855-866
-
3D arrays of microcages by two-photon lithography for spatial organization of living cells.
Lab Chip. 2019; 19: 875-884
-
3D Printing of large-scale and highly porous biodegradable tissue engineering scaffolds from poly(trimethylene-carbonate) using two-photon-polymerization.
Biofabrication. 2020; 12045036
-
Neural precursors cells expanded in a 3D micro-engineered niche present enhanced therapeutic efficacy in vivo.
Nanotheranostics. 2021; 5: 8-26
-
Fabrication of biomimetic placental barrier structures within a microfluidic device utilizing two-photon polymerization.
Int. J. Bioprint. 2018; 4: 144
-
On-chip high-definition bioprinting of microvascular structures.
Biofabrication. 2020; 13015016
-
Multiphoton microfabrication and micropatterning (MMM) – an all-in-one platform for engineering biomimetic soluble cell niches.
Biomaterials. 2021; 269120644
-
Intravital three-dimensional bioprinting. Nat.
Biomed. Eng. 2020; 4: 901-915
-
A modular approach to sensitized two-photon patterning of photodegradable hydrogels.
Angew. Chem. 2018; 130: 15342-15347
-
Guiding cell migration in 3D with high-resolution photografting.
Sci. Rep. 2022; 12: 8626
-
Engineered human blood–brain barrier microfluidic model for vascular permeability analyses.
Nat. Protoc. 2022; 17: 95-128
-
3D Microvascularized tissue models by laser-based cavitation molding of collagen.
Adv. Mater. 2022; 34: 2109823
-
Bone-on-a-chip: a microscale 3D biomimetic model to study bone regeneration.
Adv. Eng. Mater. 2021; 24: 2101467
-
From local to global matrix organization by fibroblasts: a 4D laser-assisted bioprinting approach.
Biofabrication. 2022; 14025006
-
Biomimetic 3D-printed scaffolds for spinal cord injury repair.
Nat. Med. 2019; 25: 263-269
-
A miniaturized imaging window to quantify intravital tissue regeneration within a 3D microscaffold in longitudinal studies.
Adv. Optical Mater. 2022; 10: 2101103
-
A facile multi-material direct laser writing strategy.
Lab Chip. 2019; 19: 2340-2345
-
Multimaterial 3D laser microprinting using an integrated microfluidic system. Science.
Advances. 2019; 5: eaau9160
-
Development, characterization, and applications of multi-material stereolithography bioprinting.
Sci. Rep. 2021; 11: 3171
-
Thiol-norbornene gelatin hydrogels: influence of thiolated crosslinker on network properties and high definition 3D printing.
Biofabrication. 2021; 13015017
-
Genetically engineered elastin-like recombinamers with sequence-based molecular stabilization as advanced bioinks for 3D bioprinting.
Appl. Mater. Today. 2020; 18100500
-
Elastin-like polypeptide-based bioink: a promising alternative for 3D bioprinting.
Biomacromolecules. 2021; 22: 4956-4966
-
Photo-crosslinkable recombinant collagen mimics for tissue engineering applications.
J. Mater. Chem. B. 2019; 7: 3100-3108
-
High-resolution 3D bioprinting of photo-cross-linkable recombinant collagen to serve tissue engineering applications.
Biomacromolecules. 2020; 21: 3997-4007
-
Multiphoton-polymerized 3D protein assay.
ACS Appl. Mater. Interfaces. 2018; 10: 1474-1479
-
Molecular imprinting strategies for tissue engineering applications: a review.
Polymers. 2021; 13: 548
-
Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold.
Circ. Res. 2017; 120: 1318-1325
-
Mesoscale laser 3D printing.
Opt. Express OE. 2019; 27: 15205-15221
-
Fast micron-scale 3D printing with a resonant-scanning two-photon microscope.
Addit. Manufact. 2019; 30100887
-
High-throughput multi-resolution three-dimensional laser printing.
Phys. Scr. 2018; 94015501
-
S. Sayer, Oral presentation at the International Conference on Biofabrication 2022, Talk IVM 038. https://www.biofabrication2022.org/wp-content/uploads/2022/09/Final-Program-Biofabrication-2022-1.pdf
-
Rapid assembly of small materials building blocks (voxels) into large functional 3D metamaterials.
Adv. Funct. Mater. 2020; 30: 1907795
-
Multi-foci laser microfabrication of 3D polymeric scaffolds for stem cell expansion in regenerative medicine.
Sci. Rep. 2019; 9: 1-9
Glossary
Bioink
a printable material, containing living cells, that supports the growth of the cells and the diffusion of nutrients. In vat polymerization techniques these materials are often referred to as bioresins.
Digital light processing (DLP)
a layer-by-layer light-based 3D printing technique, where each printed plane is illuminated by a single projected image, usually UV or visible wavelength range.
Electrowriting (EW)
extrusion-based 3D printing technique in which a (sub)microscale polymer jet is formed at the tip of the extrusion nozzle by applying an electric field across the nozzle and a collector plate, resulting in printing microscale fibers. Can be applied to polymer melts as well as to cell-laden hydrogels.
Extracellular matrix (ECM)
the network of macromolecules surrounding cells, with a tissue-specific organization, providing mechanical stability and stimuli to the cells, and containing growth factors and bioactive molecules.
Extrusion bioprinting
a 3D printing technique where a bioink is deposited on a substrate by pushing it through a nozzle (extrusion) following a predetermined pattern.
Multiphoton lithography (MPL)
a femtosecond laser-based high-resolution 3D printing technique. The laser is focused inside a transparent material, and nonlinear multiphoton absorption takes place only in the laser focal spot, for example leading to material polymerization.
Organ-on-a-chip
an organ model built and cultured in a microfluidic chip, to automate tasks such as nutrient supply and to facilitate optical tissue inspection.
Recombinant material
protein-based synthetic material produced by bacteria or yeasts that have been genetically modified by recombinant DNA techniques.
Stereolithography (SLA)
a UV light-based 3D printing technique in which each printed layer is scanned line by line by a laser spot.
Tension anisotropy
cells are subject to tension anisotropy when forces applied to their membrane and cytoskeleton are of different intensity in different directions.
Tomographic back-projections
a set of discretized 1D projections generated using a Radon transform. When reprojected back along the direction in which they were generated, they summatively form the cross-section of the object they were derived from (i.e., the discretized 2D solution for the inverse Radon transform).
Volumetric (bio)printing (VP)
a light-based 3D printing technique in which a 3D light dose is delivered to a volume of photoresponsive polymer, enabling layerless, rapid printing of centimeter-sized constructs. It is commonly performed utilizing a spatially modulated light source encoding visible light with tomographic backprojections.
Article Info
Publication History
Published online: December 10, 2022
Publication stage
In Press, Corrected Proof
Identification
Copyright
© 2022 The Authors. Published by Elsevier Ltd.
User License
ScienceDirect
Related Articles
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(22)00280-3?rss=yes
- 1
- 10
- 100
- 11
- 2020
- 2022
- 28
- 2D
- 3d
- 3D Printing
- 4k
- 7
- 8k
- 9
- 98
- a
- ability
- Able
- About
- ABSTRACT
- Academia
- accelerated
- accommodate
- accurate
- Achieve
- achieved
- across
- active
- added
- address
- adopted
- advanced
- advances
- ADvantage
- advantages
- After
- Agreement
- Aiming
- aims
- algorithms
- All
- all-in-one
- Allowing
- allows
- alone
- already
- alternative
- always
- among
- anatomy
- and
- animal
- animals
- Another
- answers
- Application
- applications
- applied
- Applying
- approach
- approaches
- approaching
- architecture
- AREA
- areas
- around
- arrangement
- article
- artificial
- aspect
- aspects
- Assembly
- associated
- Attainable
- attention
- authors
- automate
- available
- avoiding
- back
- Backbone
- Bacteria
- bar
- barrier
- barriers
- bars
- base
- based
- because
- becomes
- being
- below
- beneficial
- benefit
- benefits
- Better
- between
- Biofabrication
- Biomaterials
- biomedical
- Blocks
- blood
- body
- Bonds
- BONE
- boost
- boosting
- Bottom
- Breaking
- broad
- brought
- build
- Building
- built
- calculated
- Cancer
- candidates
- capabilities
- capable
- cases
- Cause
- Cells
- central
- challenge
- challenges
- change
- Channel
- channels
- characteristic
- chemical
- chip
- Chips
- chosen
- class
- classes
- Clinical
- Close
- closer
- Co-founder
- colleagues
- collector
- combination
- combine
- combining
- commercial
- commercialization
- commercially
- commonly
- Commons
- community
- comparable
- compared
- comparison
- compatible
- complementary
- complex
- complexity
- components
- compromising
- conditions
- Conference
- Connectivity
- constant
- construct
- contact
- Contactless
- context
- continuous
- contrast
- contribute
- control
- controlled
- controlling
- conventional
- Convergence
- corrected
- Corrections
- could
- Council
- create
- Creating
- creation
- critical
- crucial
- Culture
- Current
- Currently
- cycles
- data
- datapoint
- Date
- decade
- decades
- December
- decisive
- deep
- delivered
- delivering
- demonstrated
- Depending
- depends
- deposit
- deposited
- depth
- Derived
- Design
- Despite
- detailed
- determined
- develop
- Development
- device
- Devices
- different
- digital
- Dimension
- dimensions
- direct
- direction
- directly
- discuss
- discussed
- discussing
- discussions
- Disease
- diseases
- displays
- distribution
- dna
- domain
- domains
- down
- downside
- dramatically
- drive
- drug
- Drugs
- during
- each
- easily
- effect
- effectively
- effects
- efficient
- efficiently
- either
- Elaborate
- Electric
- elements
- eliminate
- embedded
- emergence
- emerging
- enable
- enables
- enabling
- encapsulated
- engineer
- Engineering
- enhanced
- ensure
- Enter
- Environment
- environments
- especially
- European
- european union
- evaluation
- Even
- events
- eventually
- evolution
- example
- examples
- exception
- exchange
- existing
- expanded
- expansion
- expected
- Exploit
- express
- extremely
- Face
- facilitate
- facing
- factors
- family
- Fashion
- faster
- Feature
- Features
- feedback
- few
- fibers
- fidelity
- field
- Fields
- Figure
- fill
- Finally
- finding
- fine
- First
- fit
- focused
- focusing
- following
- Forces
- form
- formation
- formed
- Forward
- found
- Freedom
- from
- full
- fully
- function
- functional
- funding
- further
- further development
- Furthermore
- future
- gaining
- General
- generally
- generate
- generated
- generation
- Giving
- Global
- GmBH
- goal
- good
- gradients
- grant
- great
- greatly
- Growing
- Growth
- guide
- happens
- Hardware
- having
- Heart
- helps
- here
- hierarchy
- High
- high-definition
- high-resolution
- higher
- highest
- Highlight
- highlights
- highly
- hold
- holographic
- horizon
- Horizontal
- However
- HTTPS
- human
- Hundreds
- image
- Imaging
- imperative
- important
- improve
- improved
- improving
- in
- include
- includes
- Including
- Increase
- increasing
- increasingly
- index
- industry
- inertia
- influence
- info
- information
- Innovation
- inspired
- instance
- instead
- integrated
- interconnected
- interest
- interesting
- interfaces
- internal
- International
- intrinsic
- invasion
- issue
- issues
- IT
- itself
- Key
- Label
- laboratory
- Lack
- large
- large-scale
- larger
- laser
- layer
- lead
- leading
- Led
- Level
- levels
- leveraging
- light
- likely
- LIMIT
- limitations
- Limited
- limits
- Line
- lines
- linked
- List
- literature
- living
- local
- locally
- locations
- Long
- loss
- losses
- Low
- Ltd
- maintenance
- manipulated
- Manipulation
- manufacturing
- many
- Markets
- matching
- material
- materials
- Matrix
- maximum
- mechanical
- mechanics
- mechanism
- medicine
- medium
- metamaterials
- method
- methods
- Microscope
- migration
- minimal
- minimizing
- minimum
- Mitigate
- mitigation
- model
- models
- Modifications
- modified
- modular
- molds
- molecular
- molecule
- more
- more efficient
- most
- MPL
- multiphoton
- multiple
- native
- Natural
- necessary
- Need
- needing
- network
- networks
- Nevertheless
- New
- next
- next-generation
- notably
- Notion
- number
- numbers
- object
- objects
- obstacles
- obtained
- offer
- Offers
- Old
- ONE
- online
- operation
- Opinion
- opportunities
- optics
- optimization
- order
- organization
- Organized
- Other
- Others
- Ovary
- Overcome
- overview
- panel
- panels
- papers
- parameter
- part
- particular
- past
- Patch
- Pattern
- patterns
- permission
- Personalized
- perspectives
- Pharmaceutical
- phenomenon
- physical
- Physics
- Pixel
- Place
- platform
- Platforms
- plato
- Plato Data Intelligence
- PlatoData
- points
- popularity
- possibility
- possible
- potential
- potentially
- power
- Precision
- precursor
- presence
- present
- presentation
- presented
- press
- primarily
- Problem
- process
- processes
- processing
- produce
- Produced
- Production
- Professor
- Program
- Progress
- progressively
- projected
- Projection
- projections
- promising
- promote
- promotes
- properly
- properties
- proposed
- Protein
- Proteins
- prototypes
- provide
- providing
- publications
- published
- Pushing
- question
- Questions
- quickly
- range
- rapid
- rapidly
- Rates
- ratio
- reach
- realization
- realizing
- reasonable
- receive
- recent
- recently
- Red
- redesign
- reduce
- Reduced
- reducing
- references
- referred
- refined
- regarding
- regenerative
- regions
- Regulate
- relatively
- relevance
- relevant
- reliable
- remain
- repair
- replaced
- replica
- Reported
- Reports
- representative
- representing
- represents
- reproduction
- require
- required
- Requirements
- requires
- research
- research and development
- research and innovation
- researchers
- Resolution
- resolving
- response
- restricted
- result
- resulting
- Results
- retaining
- review
- reviewed
- Rise
- Role
- round
- ROW
- sacrificing
- Safety
- same
- Scalability
- Scale
- scales
- scanning
- SCI
- screening
- Search
- seconds
- sense
- serve
- set
- setup
- several
- Shape
- Short
- should
- show
- shown
- Shows
- Signal
- signals
- significantly
- silk
- similar
- Simple
- simply
- simultaneously
- since
- single
- Size
- sizes
- small
- smaller
- So
- so Far
- solution
- Solutions
- some
- sophisticated
- Source
- Space
- Spatial
- special
- specialized
- specific
- speed
- Spot
- spring
- squares
- Stability
- stable
- stacked
- stages
- standard
- Stations
- Stem
- Step
- Steps
- Still
- strategies
- Strategy
- streamlined
- strongly
- structural
- structure
- Struggle
- studied
- studies
- Study
- subject
- Subsequently
- successful
- such
- sufficient
- suitable
- supply
- support
- Supports
- Surface
- Surrounding
- synchronization
- synthetic
- system
- Systems
- tailored
- Take
- takes
- Talk
- Target
- targets
- tasks
- techniques
- Technologies
- Technology
- terms
- test
- Testing
- The
- their
- theoretical
- therefore
- three
- three-dimensional
- Through
- throughput
- time
- time-consuming
- times
- tip
- to
- today
- together
- tool
- Toolbox
- top
- topic
- Total
- toward
- towards
- transfer
- Transform
- transition
- transitions
- Translation
- transparent
- true
- TURN
- types
- typical
- typically
- under
- underlying
- understand
- union
- unique
- unit
- unprecedented
- upcoming
- use
- usually
- Utilizing
- Valuable
- variety
- various
- VAT
- VeloCity
- versatile
- Versus
- via
- viability
- View
- visible
- volume
- volumes
- Voxels
- W
- well-defined
- What
- What is
- which
- while
- wide
- widespread
- will
- within
- without
- Work
- working
- works
- would
- writing
- X
- years
- Yield
- zephyrnet