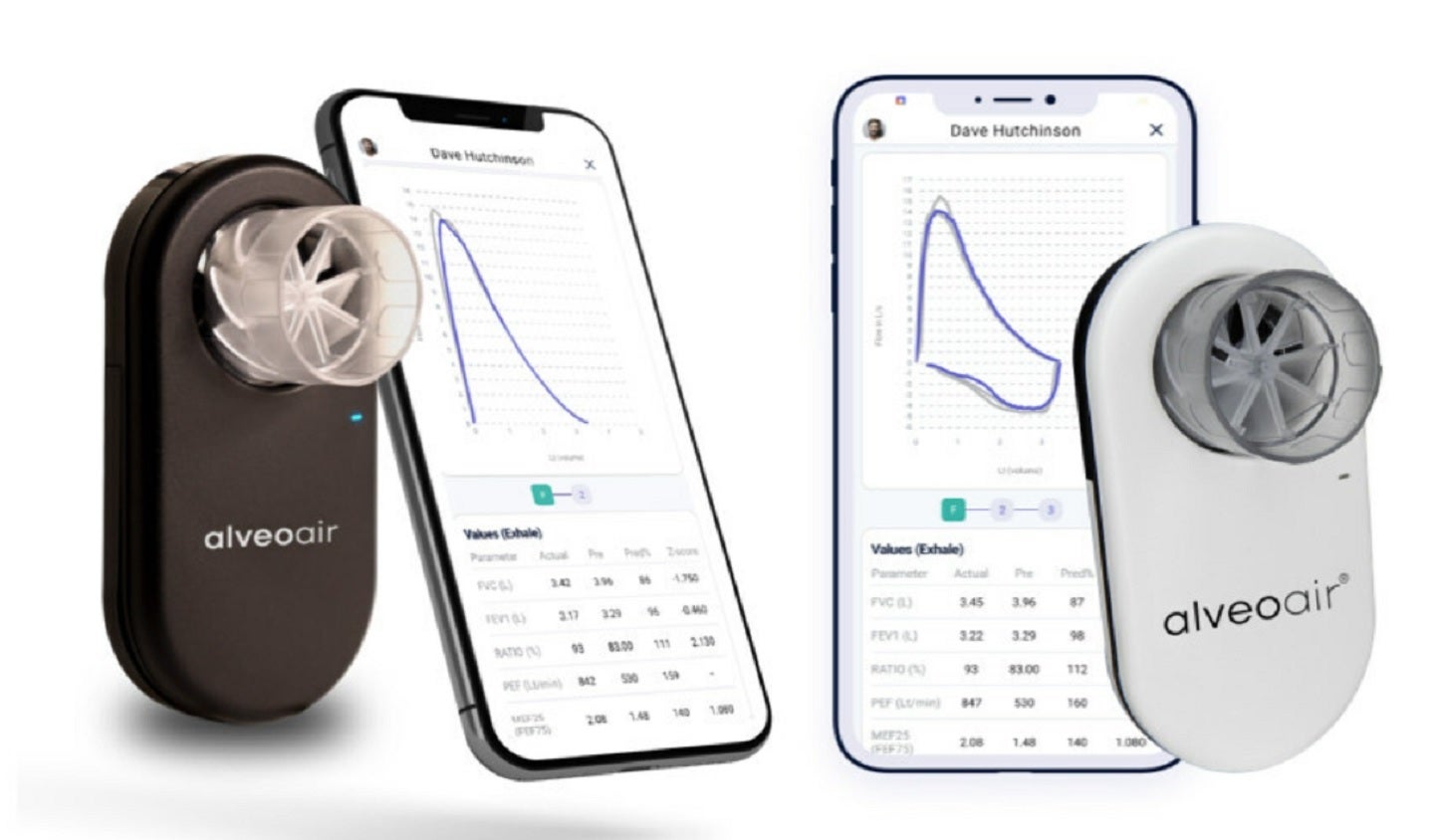
alveofit (Roundworks Technologies) has received approval from the US Food and Drug Administration (FDA) for its portable digital spirometer, alveoair.
With this approval, the company plans to start distributing the product in the US.
Earlier this year, alveofit launched its office in New York to cater to the growing US market.
It also claims that, along with the FDA approval, an alliance with AstraZeneca will expand its international business, particularly in the US and emerging economies.
The product is part of alveofit’s ecosystem, which provides real-time insights to healthcare professionals and enables timely interventions in respiratory care.
These insights can offer predictive analytics along with AI models into respiratory health and allow clinicians to prioritise cases and deliver quality care.
The CDSCO-certified alveoair is claimed to be India’s first US FDA-cleared spirometer and is adopted by more than 400 healthcare facilities across India.
In India, alveofit partnered with various companies, including NASSCOM COE, Forge Innovation & Ventures, PATH, DST and the India Sweden Innovation Centre.
NASSCOM COE CEO Sanjeev Malhotra said: “It is good to see the product mature over the last couple of years and gain acceptance among the providers.
“I congratulate and wish alveofit a global success with recent approval from the FDA, making a difference in the lives of people in India and the world over.”
Furthermore, alveofit collaborated with AIIMS Delhi for clinical research and formed an alliance with Tatvacare to extend digital respiratory care solutions to the homes of patients.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.medicaldevice-network.com/news/alveofit-fda-digital-spirometer/
- :has
- :is
- 400
- a
- acceptance
- across
- administration
- adopted
- AI
- AI models
- Alliance
- allow
- along
- also
- among
- an
- analytics
- and
- approval
- BE
- business
- by
- CAN
- care
- cases
- cater
- centre
- ceo
- claimed
- claims
- Clinical
- clinicians
- collaborated
- Companies
- company
- Couple
- credit
- Delhi
- deliver
- difference
- digital
- distributing
- drug
- economies
- ecosystem
- emerging
- enables
- Ether (ETH)
- Expand
- extend
- facilities
- fda
- First
- food
- Food and Drug Administration
- For
- forge
- formed
- from
- Gain
- Global
- good
- Growing
- Health
- healthcare
- Homes
- HTML
- HTTPS
- in
- Including
- india
- Innovation
- insights
- International
- international business
- interventions
- into
- ITS
- jpg
- Last
- launched
- Lives
- Making
- Market
- mature
- models
- more
- New
- New York
- of
- offer
- Office
- over
- part
- particularly
- partnered
- path
- patients
- People
- plans
- plato
- Plato Data Intelligence
- PlatoData
- portable
- predictive
- Predictive Analytics
- prioritise
- PRNewswire
- Product
- professionals
- providers
- provides
- quality
- real-time
- received
- receives
- recent
- research
- Said
- see
- Share
- Solutions
- start
- success
- Sweden
- Technologies
- than
- that
- The
- the world
- this
- this year
- timely
- to
- us
- various
- Ventures
- which
- will
- with
- world
- year
- years
- york
- zephyrnet