<!–
–>
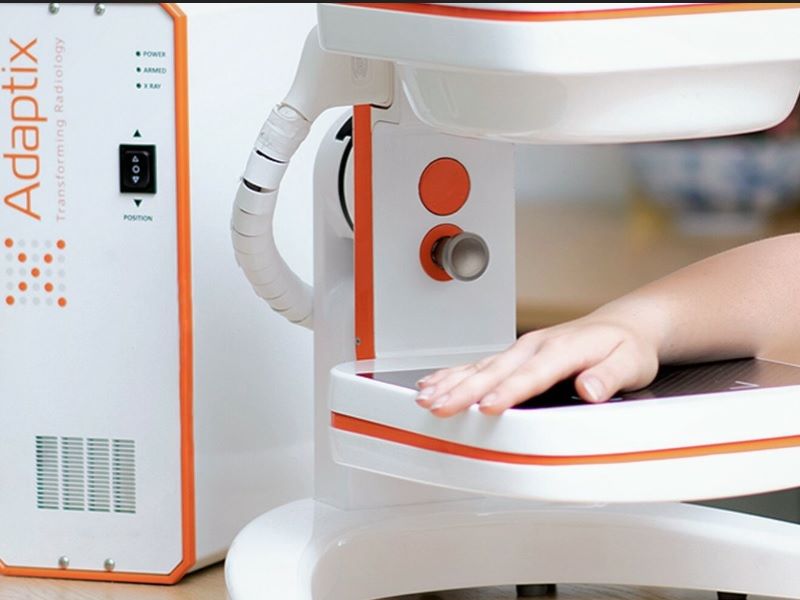
Avingtrans medical imaging technology associate firm Adaptix has received US Food and Drug Administration (FDA) 510(k) clearance for its orthopaedic medical imaging product.
The Digital Tomosynthesis Orthopaedic imaging system, which is the first Adaptix medical product, is a portable and low-dose imaging system.
The system is designed to deliver fast X-ray imaging at the point of patient care.
Adaptix has specifically developed the system to provide 3D X-ray imaging of hands, elbows and feet at a fraction of the radiation dose and per-study price of traditional CT systems.
With fewer acquisitions, accelerated patient workflow and improved diagnostic accuracy, the system offers clinicians clearer images than 2D X-ray systems.
Adaptix CEO Mark Evans said: “We are delighted to receive the 510(k) clearance, which allows this innovative technology, and our first medical product, to be marketed in the world’s largest healthcare market.
“The team are energised by achieving this milestone, and we look forward to delivering enhanced orthopaedic DT imaging systems; a dental DT imaging system; and a chest DT imaging system for Intensive Care and Emergency Department use.
“We see a future where 3D travels to the patient at the point-of-care throughout hospitals, clinics and primary care transforming patient pathways, improving patient experience and reducing the cost of care delivery.”
In 2021, Avingtrans invested £4m to purchase an 11.9% interest in Adaptix. Last December, the company invested a further £2m through a convertible loan.
Adaptix is already offering services to customers in the veterinary and industrial markets.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.medicaldevice-network.com/news/adaptix-orthopaedic-medical-imaging-system/
- 11
- 2021
- 2D
- 3d
- a
- accelerated
- accuracy
- achieving
- acquisitions
- Ad
- administration
- allows
- already
- and
- Associate
- care
- ceo
- clearer
- clinicians
- company
- Cost
- credit
- Customers
- December
- delighted
- deliver
- delivering
- delivery
- Department
- designed
- developed
- digital
- drug
- emergency
- enhanced
- experience
- FAST
- fda
- Feet
- Firm
- First
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- Forward
- fraction
- further
- future
- GlobalData
- Hands
- healthcare
- hospitals
- HTTPS
- images
- Imaging
- improved
- improving
- in
- industrial
- innovative
- interest
- invested
- largest
- Last
- loan
- Look
- mark
- Market
- Markets
- medical
- medical imaging
- milestone
- offering
- Offers
- patient
- patient care
- plato
- Plato Data Intelligence
- PlatoData
- PLC
- Point
- price
- primary
- Product
- provide
- purchase
- Radiation
- receive
- received
- reducing
- Said
- Services
- Share
- Size
- specifically
- system
- Systems
- team
- Technology
- The
- Through
- throughout
- to
- traditional
- transforming
- travels
- unit
- use
- veterinary
- which
- workflow
- world’s
- x-ray
- zephyrnet