<!–
–>

The US Food and Drug Administration (FDA) has issued a 510(k) clearance to 23andMe to include an additional 41 variants of BRCA1 and BRCA2 genes as part of its BRCA1/BRCA2 (Selected Variants) Genetic Health Risk Report.
23andMe first received FDA clearance for at-home genetic testing of three variants of BRCA1 and BRCA2 genes in 2018. The company plans to release the updated report by the end of next year.
The news led to a spike in the 23andMe stock price, it was up by more than 18% in pre-market trading on 1 September, compared to the market close on the previous day. 23andMe’s market cap stands at $519.74m.
The BRCA1 and BRCA2 genes have been associated with an increased risk of developing breast, ovarian, prostate, and pancreatic cancer.
The US-based company also received the first-ever Predetermined Change Control Plan (PCCP), which outlines the protocols and acceptance criteria to validate BRCA1 and BRCA2 variants. The PCCP allows 23andMe to add other validated BRCA1 and BRCA2 variants and associated cancer risk information to its BRCA Genetic Health Risk Report without the need to obtain premarket reviews.
The company intends to add the new variants to the BRCA1/BRCA2 (Selected Variants) reports for its new and existing customers. The report will also include an education module for these variants to inform the customers of their results.
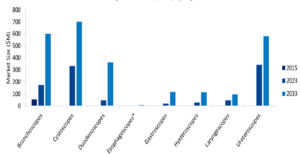
Genetic testing is a growing area of research. There are over 500 products in development for genetic testing, according to GlobalData. Of these, 279 are in the late stages of development, with GlobalData predicting these to gain approval within the next decade.
Other reports included as part of 23andMe’s Genetic Health Risk Report are hereditary prostate cancer (HOXB13-Related) report, late-onset Alzheimer’s disease report, MUTYH-Associated Polyposis report for colorectal polyps and cancer, and Parkinson’s disease report.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.medicaldevice-network.com/news/23andme-expands-cancer-at-home-genetic-report/
- :has
- :is
- $UP
- 1
- 2018
- 220
- 500
- a
- acceptance
- According
- Ad
- add
- Additional
- administration
- allows
- also
- Alzheimer’s
- an
- and
- approval
- ARE
- AREA
- AS
- associated
- At
- been
- by
- Cancer
- cap
- change
- clearance
- Close
- company
- compared
- control
- credit
- criteria
- Customers
- day
- decade
- developing
- Development
- Disease
- drug
- Education
- end
- existing
- expands
- fda
- First
- first-ever
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- For
- Gain
- GlobalData
- Growing
- Have
- Health
- HTML
- HTTPS
- image
- in
- include
- included
- increased
- inform
- information
- intends
- Issued
- IT
- ITS
- jpg
- Late
- Led
- Market
- Market Cap
- module
- more
- Need
- New
- next
- obtain
- of
- on
- Other
- outlines
- Parkinson’s Disease
- part
- plan
- plans
- plato
- Plato Data Intelligence
- PlatoData
- predicting
- previous
- price
- Products
- protocols
- received
- release
- report
- Reports
- research
- Results
- Reviews
- Risk
- selected
- September
- Share
- shutterstock
- Size
- spike
- stages
- stands
- stock
- Testing
- than
- The
- their
- There.
- These
- this
- three
- to
- Trading
- unit
- updated
- us
- US Food
- VALIDATE
- validated
- was
- which
- will
- with
- within
- without
- year
- zephyrnet