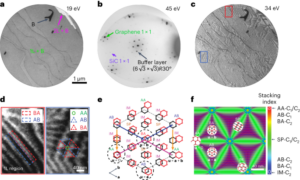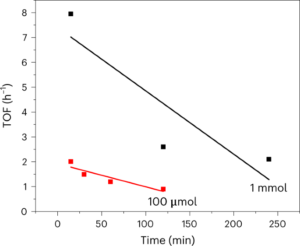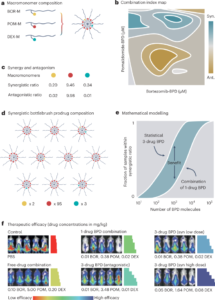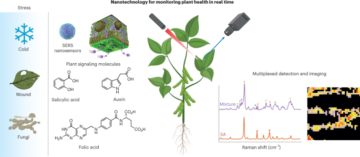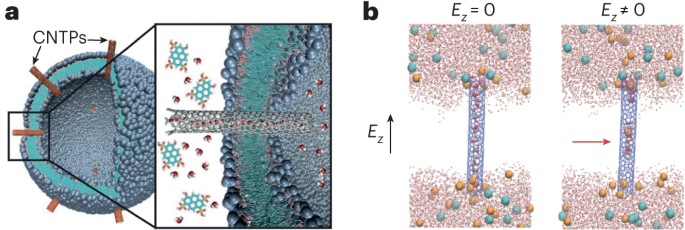
Nanoscale fluid dynamics, or nanofluidics, is an emerging field of research where the continuum of hydrodynamics meets the atomic nature of matter1. Understanding the laws that govern liquid flows at such molecular scales is of key practical importance: they determine the performance of seawater desalination membranes, and the way ions permeate through the pores in our cells. For electron flows in solids, it is now history that scale reduction yields qualitatively new behaviour, which is at the basis of the whole nanoelectronics industry. Yet, for water flows and ion transport therein, the laws established at the macroscopic scale hold surprisingly well at the molecular scale. A water molecule is about 0.3 nm in diameter; yet the Navier–Stokes equation — the basic equation of hydrodynamics — still holds in 1-nm-wide channels2. Now, writing in Nature Nanotechnology, Noy, Blanckschtein and coworkers3 demonstrate that the Nernst–Einstein relation — a fundamental law that governs ion dynamics in solution — breaks down in extremely narrow carbon channels that confine water and ions to a single one-dimensional chain.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.nature.com/articles/s41565-022-01281-3
- 1
- 2021
- 2022
- 39
- a
- About
- Anchor
- and
- basic
- basis
- Break
- breaks
- carbon
- Cells
- chain
- channels
- Continuum
- demonstrate
- Determine
- down
- dynamics
- emerging
- established
- Ether (ETH)
- extremely
- field
- Flows
- Fluid dynamics
- fundamental
- governs
- history
- hold
- holds
- HTTPS
- importance
- in
- industry
- IT
- Key
- Law
- Laws
- LINK
- Liquid
- Meets
- molecular
- molecule
- Nature
- New
- performance
- plato
- Plato Data Intelligence
- PlatoData
- Practical
- relation
- research
- Scale
- scales
- single
- solution
- Still
- such
- The
- the Law
- therein
- Through
- to
- transport
- understanding
- Water
- which
- writing
- yields
- zephyrnet